Introduction
The United States produced $1.28 billion worth of peanuts in 2019 of which Georgia produced 51% of the total production (USDA-NASS 2021). Peanut is susceptible to weed competition due to slow canopy establishment, prostrate growth habit, and wide critical period for weed control from 3 to 8 weeks after planting (Burke et al. 2007; Everman et al. 2008). Georgia-06G is the dominant peanut cultivar planted in the southeast and in 2020, 87% of the acres grown for certified peanut seed available for sale to growers was Georgia-06G (Anonymous, 2020a). Peanut is commonly grown in rotation with cotton in the region and therefore, similar weed issues between these systems persist. This includes Palmer amaranth (Amaranthus palmeri S. Watson) which has been documented to be resistant to multiple herbicide modes of action making its control difficult (Heap 2021). To minimize yield loss from weeds, preemergence (PRE) herbicides are frequently used in peanut to inhibit weed germination and provide residual weed control (Grichar et al. 2001). In response to resistance issues, producers have continued to integrate PRE herbicides into their herbicide programs to minimize weed emergence.
One commonly used PRE herbicide in peanut is flumioxazin, a WSSA Group 14 protoporphyrinogen oxidase (PPO) inhibitor. Flumioxazin provides long residual activity, a high level of efficacy, and an overall fit into weed management programs by expanding modes of action applied PRE in peanut (Askew et al. 1999; Clewis et al. 2002, Main et al. 2003). It has been shown to provide > 85% control in peanut of various problematic weeds without affecting pod grade or disease incidence. Furthermore, flumioxazin applied PRE controlled 99% of Palmer amaranth that had resistance to POST applied PPO inhibitors, further emphasizing its importance to peanut production systems where Palmer amaranth is problematic (Umphres et al. 2018; Wilcut et al. 2001).
Metolachlor, a 50/50 mixture of R/S stereoisomers, was first registered for use in peanut in 1980 (H. McLean, pers. communication). In 1997, S-metolachlor, a 12:88 mixture of R/S stereoisomers was commercialized (H. McLean, pers. communication). Formulations with a greater concentration of S-stereoisomers are more active and can be applied at lower rates controlling annual grasses (Digitaria, Setaria, and Panicum spp.) and small-seeded broadleaf weeds (Amaranthus and Solanum spp.) (Anonymous 2020b; O’Connell et al. 1998). When applied at equivalent rates, no differences in performance between S-metolachlor and metolachlor have been observed in peanut (Grichar et al. 2001). In general, and when applied at labeled rates, peanut emergence can be delayed and plant stunting was observed following soil application of metolachlor. However, yields were not negatively impacted (Cardina and Swann, 1988; Johnson et al.1993, 1994; Wehtje et al. 1988). Others have suggested that including S-metolachlor in a peanut weed control program can result in a positive yield response (Clewis et al. 2007; Grichar et al. 2001).
Despite flumioxazin and S-metolachlor’s utility in a peanut production system, peanut injury after emergence has been variable, with injury ranging from minimal to >60% (Burke et al. 2002; Grey et al. 2005; Johnson et al. 2006; Main et al. 2003; Price et al. 2004; Wilcut et al. 2001). Previous publications have identified increases in injury as possibly relating to cold, wet conditions which slow plant metabolism, and increase herbicide availability for uptake (Askew et al. 1999; Burke et al. 2002; Grichar et al. 2004). During heavy rainfall, flumioxazin available in the soil water can splash onto the plant, allowing for foliar uptake and increasing peanut injury. In much of the southeastern US peanut-growing regions, heavy rainfall can occur during peanut emergence. These rainfall events increase the potential for early-season injury, however, the impact of this early-season injury on peanut injury, growth, and end-of-season yields has not been determined.
Early research suggested that J-rooting is partially caused by soil applications of metolachlor (Cardina and Swann 1988). However, there has not been additional research to connect J-rooting directly to applications of metolachlor/S-metolachlor or any other herbicide. J-rooting is a phenomenon where peanut taproot does not continue to grow downward but instead growth has inverted geotropism. The taproot then grows up toward the soil surface resulting in a “J” shaped root. The cause of J-rooting is not well understood but the appearance of J-rooting is often associated with plants under stress. Historically, there have always been grower concerns about the use of soil-applied metolachlor/S-metolachlor in peanut as it has anecdotally been connected to J-rooting. While the effects of S-metolachlor on peanut grown and yield have been addressed, there has not been additional research conducted to connect J-rooting to applications of S-metolachlor or any other herbicide.
Both flumioxazin and S-metolachlor require activating rainfall when used PRE in peanut. However, heavy spring rains following applications of PRE herbicides can result in significant peanut injury. In the peanut-growing regions of Georgia, rainfall exceeding 15 cm during and following planting can occur (Georgia Weather Network, 2021). Coupling heavy rainfall and the prostrate growth habit of peanut, young plants often make contact with herbicide-treated soil or are exposed at the rooting zone from downward herbicide movement with rainfall. Therefore, the objective of this study was to evaluate the effect of simulated high rainfall on peanut injury, J-rooting, and yield after applications of PRE combinations of flumioxazin and S-metolachlor.
Materials and Methods
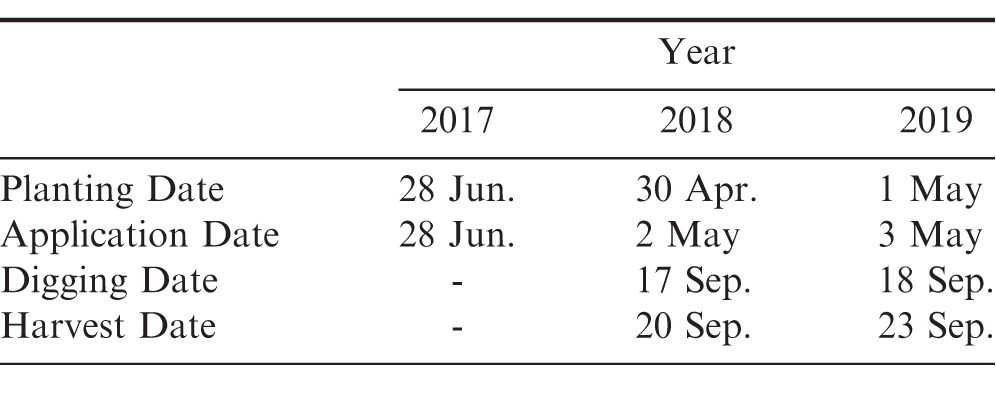
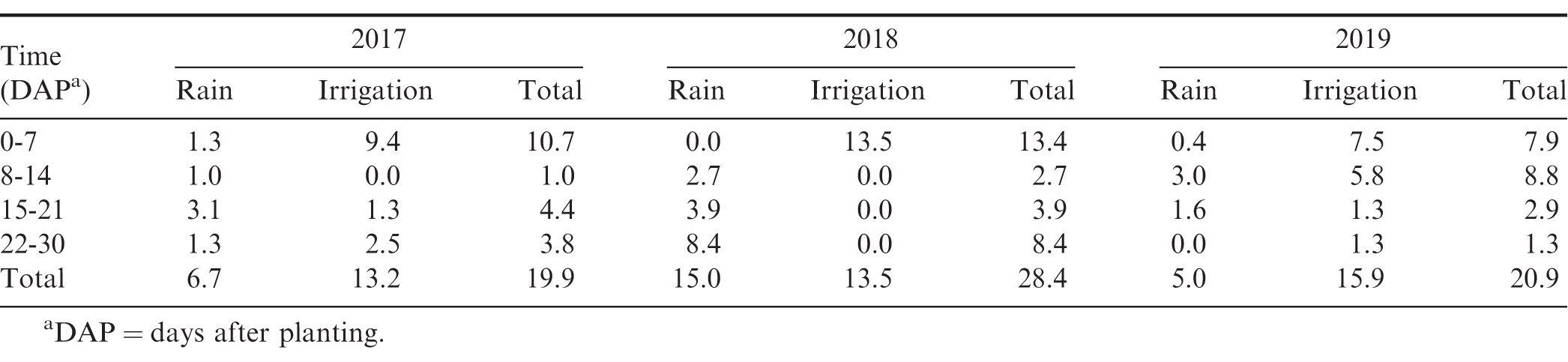
Small plot field trials were conducted in 2017 through 2019 at the University of Georgia Ponder Research Farm located near Ty Ty, Georgia (31.507654°N, -83.658395°W). The soil type was a Fuquay sand with 96% sand, 0% silt, 4% clay, 0.57% organic matter, and a pH of 6.0. Conventional tillage practices were used and peanut was planted using a vacuum planter calibrated to deliver 18 peanut seed/m at a depth of 5 cm (Monosem Precision Planters, 1001 Blake St., Edwardsville, KS). Peanut (cv. GA-06G) was planted in a twin-row pattern (90 cm X 22 cm spacing) with a plot size of 7.6 m X 0.9 m. Planting, application, and harvest dates are presented in Table 1. Rainfall and supplemental irrigation totals for the first 30 days after planting (DAP) are presented in Table 2. These 30 DAP moisture totals ranged between 19.9 and 28.4 cm.
Treatments were arranged in a randomized complete block design with a 3 (flumioxazin rates) by 4 (S-metolachlor rates) factorial arrangement. Flumioxazin rates were 0, 0.11 and 0.22 kg ai/ha. S-metolachlor rates were 0, 1.07, 1.40, and 2.80 kg ai/ha. Normal use rates of these herbicides in peanut production are 0.11 and 1.07 kg ai/ha for flumioxazin and S-metolachlor, respectively (Anonymous 2016, Anonymous 2020b). Herbicide treatments were replicated 3 to 4 times and were applied using a CO2 – pressurized backpack sprayer calibrated to deliver 140 L/ha at 4.8 km/hr. Plots were maintained weed-free throughout the season using a combination of herbicides (pendimethalin, diclosulam, imazapic, and 2,4-DB) and hand-weeding. Peanut density, whole-plant (above + below ground) fresh weight biomass, and J-rooting was determined by hand-harvesting entire peanut plants from a 1 m section of row at 21 DAP. Visual estimates of peanut injury were made using a scale from 0 (no injury) to 100% (complete plant death) and collected 10 days after treatment (DAT) and 50 DAT. Peanut yield data were obtained by mechanical harvesting at maturity. Yield data were not collected in 2017 due to a very late planting date.
Data were subjected to ANOVA using PROC GLIMMIX in SAS, version 9.4 (SAS Institute, Cary, NC) to determine if year, flumioxazin rate, and S-metolachlor rate influenced peanut injury, stand density, whole-plant fresh biomass, J-rooting, and yield. Peanut injury, density, biomass, J-rooting, and yield were set as the response variables with year and replication within year included in the model as random factors. Year was not significant so all data were combined over years. Flumioxazin rate by S-metolachlor rate interactions were only significant for peanut density and whole-plant fresh weight biomass, therefore other response variables were analyzed by the main factor of herbicide. All P-values for tests of differences between least-squares means were compared and separated using the Tukey-Kramer method (P<0.10).
Results & Discussion
Peanut injury
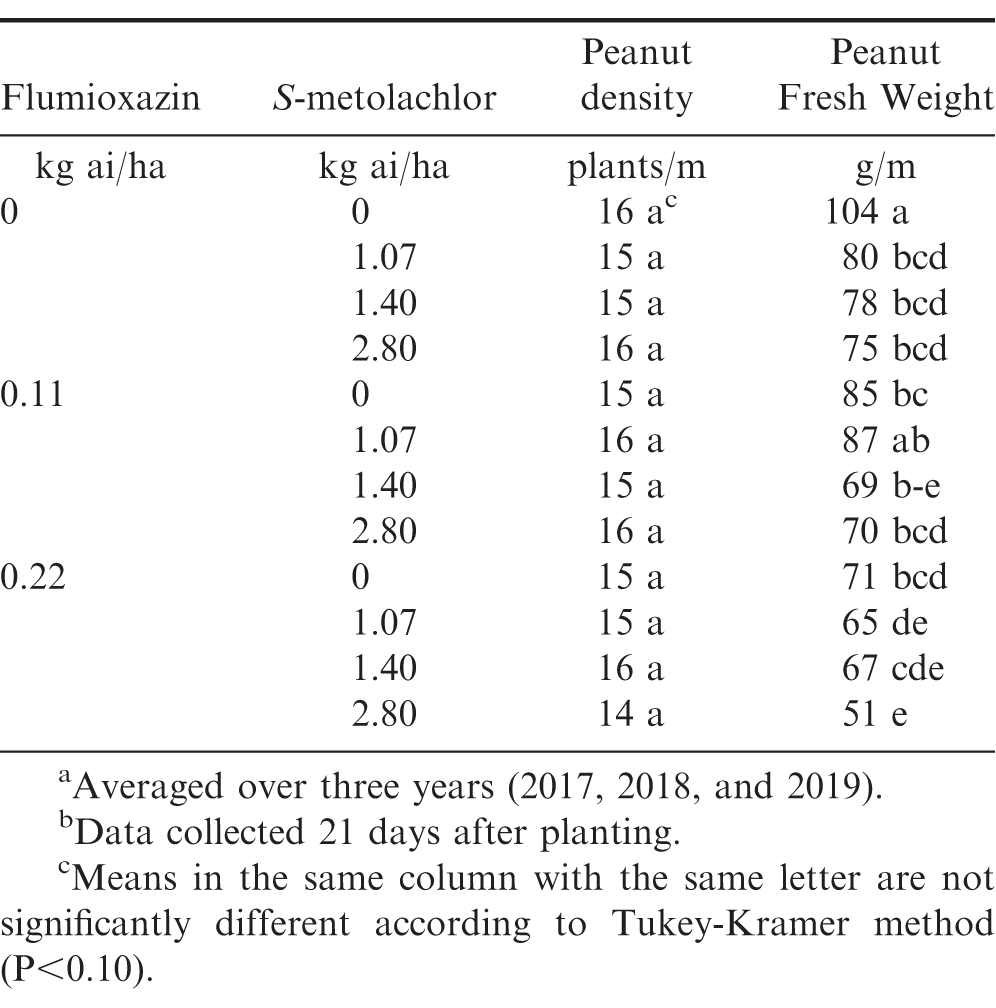
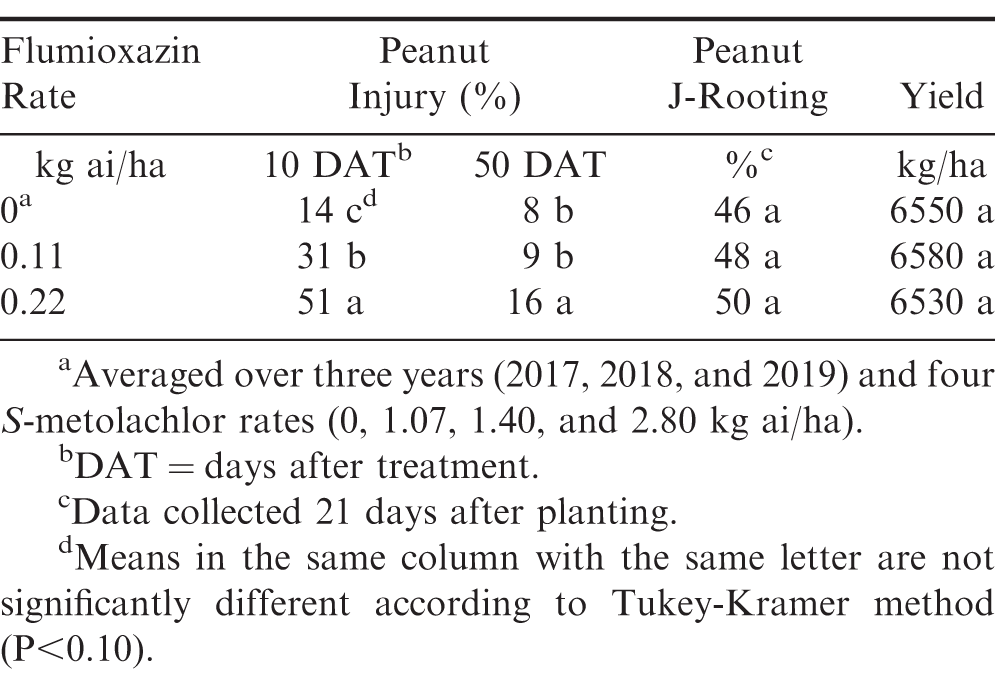
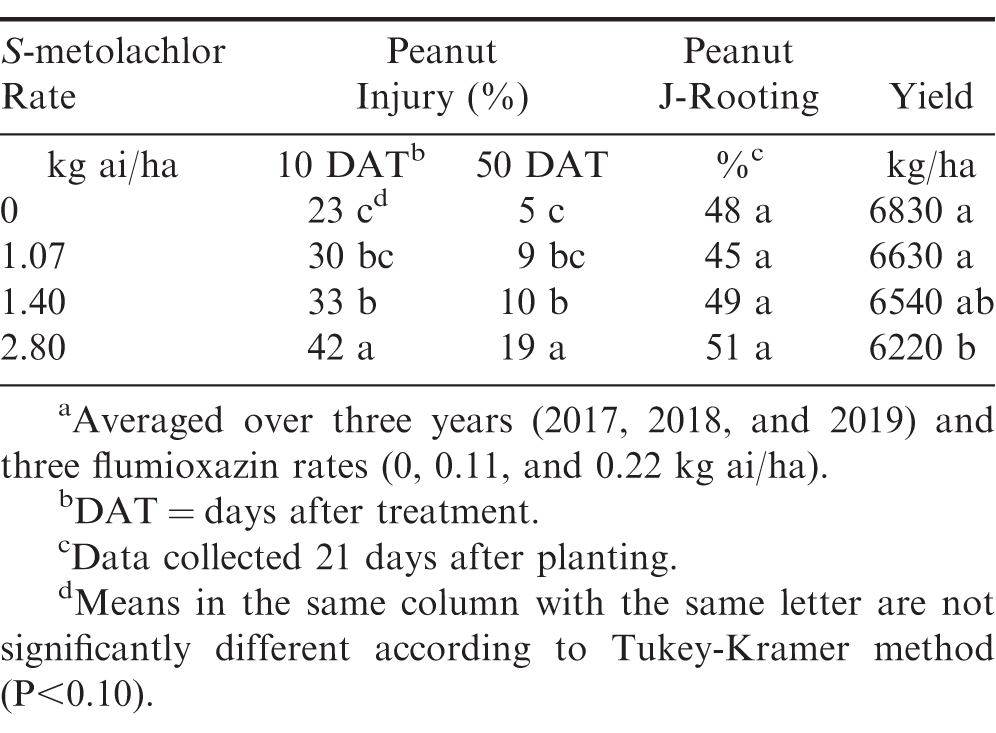
At all rates, flumioxazin and S-metolachlor did not affect peanut emergence across all years (Table 3). Peanut plants were stunted, as indicated by reduced fresh weights in herbicide-treated plots at 21 DAP when compared to the untreated check. Fresh weights of peanut subjected to normal rates of flumioxazin (0.11 kg ai/ha) and S-metolachlor (1.07 kg ai/ha) were similar to the untreated check (Table 3). When the highest rate of both flumioxazin and S-metolachlor were applied to peanut, fresh weights were reduced by 49%. Peanut injury was transient in all years with the greatest injury occurring at 10 DAT. Injury increased with increasing herbicide rate for both flumioxazin and S-metolachlor (Tables 4 and 5). By 50 DAT, injury was less than 20% for all treatments. Despite increased moisture in this study, injury ratings did not exceed those reported in other studies for flumioxazin and S-metolachlor (Askew et al. 1999; Burke et al. 2002; Cardina and Swann 1988). Increasing injury with increasing flumioxazin rate has been noted in other studies (Askew et al. 1999; Burke et al. 2002; Jordan et al. 2009). In these studies, peanut injury was transient, even with increased injury occurring. For S-metolachlor, injury responses increased with increasing herbicide rate but fell below 20% for all rates by 50 DAT. Injury for S-metolachlor was also noted in previous studies (Cardina and Swann 1988), however, rates used in that study were greater than those used in this study and exceed labeled rates for application of this product (Anonymous, 2020b)
J-rooting
J-rooting was not affected by any herbicide. At the time of plant harvest (21 DAP), J-rooting was present in all plots including the untreated check at rates >45%. A previous study connected the J-rooting phenomenon to the use of S-metolachlor (Cardina and Swann 1988). However, the herbicide rate of 6.7 kg ai/ha used in the present study is far above rates that are typically used in production fields. Although studies have noted root malformations with the use of S-metolachlor in other below-ground crops (Abukari et al. 2015; Meyers et al. 2012), this three-year study had J-rooting at the same level in plots treated with S-metolachlor and flumioxazin as the untreated check. The present study indicates that J-rooting is not caused by typical field use rates of S-metolachlor or flumioxazin, even under high moisture conditions that could move these herbicides into the root zone. Therefore, this phenomenon may be influenced by other seed, environmental, or edaphic conditions.
Peanut yield
Despite rates causing up to 51% injury at 10 DAT, flumioxazin did not affect yield (Table 4). This result is similar to previous literature, indicating that flumioxazin does not negatively impact yield even when high levels of early-season injury occurred (Askew et al. 1999; Burke et al. 2002; Main et al. 2003). However, yields were significantly reduced by the 2.80 kg ai/ha rate of S-metolachlor. This rate exceeds current labeled rates by 2.6X (Anonymous, 2020b). Negative yield impacts from metolachlor at labeled rates were not observed and are supported by previous literature (Cardina and Swann 1988; Johnson et al. 1993, 1994; Wehjte et al. 1988). The results of one study suggested that tank-mixing S-metolachlor and flumioxazin PRE improved peanut yield over S-metolachlor alone (Clewis et al. 2007). In another study, S-metolachlor increased yields due to eliminating competition from yellow nutsedge (Cyperus esculentus) (Grichar et al. 2001).
Summary and Conclusions
Results from this study support the continued use of labeled rates of flumioxazin and S-metolachlor to combat weeds in peanut. Flumioxazin and S-metolachlor both caused early-season injury in peanut, and injury for both herbicides was directly related to the application rate. The early-season injury from flumioxazin did not have a negative impact on yield, while yield was negatively impacted by S-metolachlor at rates above the recommended labeled rates were applied. As “Georgia-06G” is grown throughout much of the peanut growing regions of the US, injury caused by these products can be tolerated by growers when heavy rainfall does occur. Furthermore, during all three years of this study, J-rooting was not increased by the application of S-metolachlor or flumioxazin. This indicates that J-rooting is caused by other factors. Additional research into the causes J-rooting should be investigated to include other biotic or abiotic factors.
Acknowledgements
The authors wish to thank Dwayne Dales, Charlie Hilton, and Tim Richards for their technical expertise.
Literature Cited
Abukari, I.A., Shankle, M.W. Reddy. K.R. 2015. Sweetpotato [Ipomoea batas (L.) Lam.] response to S-metolachlor and rainfall under three temperature regimes. Am J Plant Sci 6: 702– 717
Anonymous. 2020 a. Peanut & Soybean Buyers Guide. Georgia Crop Improvement Association. Online. Accessed at http://www.georgiacrop.com/fullpanel/uploads/files/2021-peanut—soybean-buyers-guide-final-00002.pdf. Accessed: February 26, 2021
Anonymous. 2020 b. Dual II Magnum herbicide product label. Syngenta Crop Protection publication No. 818A-L1U 1020. Greensboro, NC. 54 p.
Anonymous. 2016. Valor SX herbicide product label. Valent U.S.A Corporation publication No. 2016-VSX-0001. Walnut Creek, CA. 31 p.
Askew, S.D., Wilcut, J.W. Cranmer. J.R. 1999. Weed management in peanut (Arachis hypogaea) with flumioxazin preemergence. Weed Technol. 13: 594– 598.
Burke, I.C., Askew, S.D. Wilcut. J.W. 2002. Flumioxazin systems for weed management in North Carolina peanut (Arachis hypogaea)1. Weed Technol. 16: 743– 748.
Burke, I.C., Schroeder, M. Thomas, W.E. and Wilcut. J.W. 2007. Palmer amaranth interference and seed production in peanut. Weed Technol. 21: 367– 371.
Cardina J. and Swann. C.W. 1988. Metolachlor effects on peanut growth and development. Peanut Sci. 15: 57– 60.
Clewis, S.B., Askew S.D., and Wilcut. J.W. 2002. Economic assessment of diclosulam and flumioxazin in strip- and conventional-tillage peanut. Weed Sci. 50: 378– 385
Clewis, S.B., Everman, W.J. Jordan, D.L. and Wilcut. J.W. 2007. Weed management in North Carolina peanuts (Arachis Hypogaea) with S-metolachlor, diclosulam, flumioxazin, and sulfentrazone systems. Weed Technol. 21 (3), 629– 635.
Everman, W.J., Clewis, S.B. Thomas, W.E Burke, I.C. and Wilcut. J.W. 2008. Critical period of weed interference in peanut. Weed Technol. 22: 63– 67.
Georgia Weather Network. 2021. Online. Accessed at: http://www.georgiaweather.net/. Accessed: February 26, 2021
Grey, T.L., and Wehtje. G.R. 2005. Residual herbicide weed control systems in peanut. Weed Technol. 19: 560– 567.
Grichar, W.J., Lemon, R.G. Brewer, K.D. and Minton. B.W. 2001. S-Metolachlor compared with metolachlor on yellow nutsedge (Cyperus esculentus) and peanut (Arachis hypogaea). Weed Technol. 15: 107– 111.
Grichar, W.J., Besler, B. A. Dotray, P. A. Johnson, W. C.and Prostko. E. P. 2004. Interaction of flumioxazin with diemthenamid or metolachlor in peanut (Arachis hypogaea L.) Peanut Sci. 31: 12– 16.
Heap, I. 2021. The International Herbicide-Resistant Weed Database. Online. Accessed at www.weedscience.org. Accessed: February 19, 2021.
Johnson, W.C., Prostko, E.P. and Mullinix, B.G., 2006. Phytotoxicity of delayed applications of flumioxazin on peanut (Arachis hypogaea). Weed Technol. 20: 157– 163.
Johnson, W.C., Colvin, D.L. and Mullinix, B.G. 1993. Comparative response of three peanut cultivars to multiple herbicide applications. Peanut Sci. 20: 17– 20.
Johnson, W.C., Brenneman, T.B. and Mullinix B.G. 1994. Chloroacetamide herbicides and chlorimuron do not predispose peanut (Arachis hypogaea) to stem rot (Sclerotium rolfsii). Peanut Sci. 21: 126– 129.
Jordan, D.L., Lancaster, S.H. Lanier, J.E. Lassiter, B.R. and Johnson. P.D. 2009. Peanut and eclipta (Eclipta prostrata) response to flumioxazin. Weed Technol. 23: 231– 235.
Main, C.L., Ducar, J.T. Whitty, E.B. and Macdonald. G.E 2003. Response of three runner-type peanut cultivars to flumioxazin. Weed Technol. 17: 89– 93.
Meyers, S.L., Jennings, K.M. and Monks. D.W. 2012. Response of sweetpotato cultivars to S-metolachlor rate and application time. Weed Technol. 23: 474– 479.
O’Connell, P.J., Harms, C.T. and Allen. J.R. 1998. Metolachlor, S-metolachlor, and their role within sustainable weed-management. Crop Prot. 17: 207– 212.
Price, A.J., Wilcut, J.W. Cranmer. J.R. 2004. Physiological behavior of root-absorbed flumioxazin in peanut, iveyleaf morningglory (Ipomoea hederacea), and sicklepod (Senna obtusifolia). Weed Sci. 52: 718– 724
Umphres, A.M., Steckel, L.E. Mueller. T.C. 2018. Control of protoporphyrinogen oxidase inhibiting herbicide resistant and susceptiblePalmer amaranth (Amaranthus palmeri) with soil-applied protoporphyrinogen oxidase-inhibiting herbicides. Weed Technol 32: 95– 100.
[USDA-NASS] US Department of Agriculture (2021) Crop Values 2020 Summary. National Agricultural Statistics Service. Washington, DC. 502 p.
Wilcut, J.W., Askew, S.D. Bailey, W.A. Spears, J.F. Isleib. T.G. 2001. Virginia market-type peanut (Arachis hypogaea) cultivar tolerance and yield response to flumioxazin preemergence. Weed Technol. 15: 137– 140.
Wehjte, G., Wilcut, J.W. Hicks, T.V. and McGuire. J. 1988. Relative tolerance of peanuts to alachlor and metolachlor. Peanut Sci. 15: 53– 56.
Notes
- First author: Assistant Professor, Dept. of Crop & Soil Sciences, University of Georgia, Athens, GA 30602 [^]
- Second and third authors: Graduate Research Assistant and Professor, Dept. of Crop & Soil Sciences, University of Georgia, Tifton, GA 31793. [^] *Corresponding author Email: Nicholas.Basinger@uga.edu
Author Affiliations