Introduction
Stem rot, also known as white mold (WM) and southern blight, are all common names for the same peanut (Arachis hypogaea L.) disease caused by the soil-borne fungus, Sclerotium rolfsii Sacc. Tomato spotted wilt disease is caused by the tospovirus Tomato spotted wilt virus (TSWV).
Both stem rot and TSWV are currently major disease problems not only in the southeast but also in the major peanut production areas of the U.S. and throughout other parts of the world. In Georgia (2010-2012), damage and cost of control for these two diseases have been estimated to be among the highest losses ranging from $50 to $60 million annually (Kemerait, 2012, 2013, 2014).
Branch and Csinos (1987) proposed the use of both low disease incidence and high yield performance in evaluating peanut genotypes for S. rolfsii resistance among all four of the U.S. market types: valencia, spanish, runner, and virginia. Branch and Brenneman (1993) then reported stem rot resistance in the late season maturing cultivar ‘Southern Runner’ (Gorbet et al., 1987), and equal or greater resistance was noted in the medium-maturing advanced Georgia breeding line, GA T-2741, which was subsequently released as ‘Georgia Browne’ (Branch, 1994).
Gorbet et al., (2004) reported that ‘C-99R’ (Gorbet and Shokes, 2002) and other relatively later maturing peanut genotypes, 150 or greater days after planting (DAP) to maturity, in the Florida breeding program were a more reliable source of stem rot (white mold) resistance as compared to the early (ca. 125 DAP) and medium maturity (ca. 135 to140 DAP) genotypes. However, ‘AP-3’ (Gorbet, 2007) a medium maturing cultivar was likewise reported in this same study to have the highest yield and lowest stem rot (white mold) disease ratings among the medium maturity peanut lines (Gorbet et al., 2004). Recently, the medium-late (ca. 140 to 145 DAP) maturing cultivar ‘Florida-07’ (Gorbet and Tillman, 2009) was released with resistance to both TSWV and stem rot. Branch and Brenneman (2009) reported that the combination of stem rot and TSWV resistance and highest yield over multiple years was consistently found in the medium-maturity, runner-type peanut cultivars, ‘Georgia-07W’ (Branch and Brenneman, 2008), ‘Georgia-03L’ (Branch, 2004), and ‘AP-3’.
Consequently, new and improved resistant cultivars are continually needed to minimize damage, reduce chemical control costs, and increase peanut yield. The objective of this research was to evaluate several recently released peanut genotypes for combined resistance to both stem rot (white mold) and TSWV.
Materials and Methods
From 2010 to 2013, several peanut cultivars and breeding lines were compared to one or three of the previously reported (Branch and Brenneman, 2009) runner-type check cultivars (AP-3, Georgia-03L, and Georgia-07W). Field test evaluations were conducted each year on a Tifton loamy sand soil type (fine-loamy, siliceous, thermic, Plinthic Kandiudult) at the Gibb’s research farm near the Coastal Plain Experiment Station. The field site has a long history of continuous peanut production and a very high incidence of stem rot (WM). Plots consisted of two rows 6.1m long x 1.8 m wide, and six peanut seed were planted per 30.5 cm of row. Early-planting dates of 19 April 2010, 15 April 2011, 16 April 2012, and 11 April 2013 were used to increase tomato spotted wilt and stem rot disease pressure (Tillman et al., 2007 and Culbreath et al., 2009). Irrigation was applied as needed during drought stress periods to ensure host-plant development. Recommended production practices were followed throughout each growing season, except no fungicides with known activity against S. rolfsii were used. Individual genotypes were dug and inverted according to hull-scrape determination based upon adjacent border plots (Williams and Drexler, 1981). After harvest, peanut pods were dried with forced warm air to approximately 6% moisture, and cleaned over a screen table before weighing for yield.
Incidence of TSW was first assessed at mid-season (ca. 60 DAP) when TSW is usually the primary disease present. At mid-to-late season (ca. 100 DAP), the combination of TSW and stem rot (WM) incidence was also assessed, which generally included predominantly TSW and some stem rot (WM). Prior to digging (ca. 140 DAP), the incidence of stem rot and TSW combined was again assessed, which generally included a higher proportion of stem rot than TSW. Immediately after digging and inverting, the incidence of only stem rot was also assessed among the different genotypes. This is the most definitive stem rot rating as signs and symptoms of this disease are often below ground. For both TSWV and stem rot assessments, the disease incidence was determined by counting the number of 30.5 cm-sections of a row with one or more infected plants and converting to a percentage of total row length for each plot.
A randomized complete block design was used each year with six replications. Data from each test was statistically compared using ANOVA, and Waller-Duncan’s T-test (k-ratio = 100) was used for mean separation in SAS (SAS Institute, Inc., Cary, NC).
Results and Discussions
The 10 total peanut genotypes used each year in this disease evaluation study were not the same for all four years (2010 to 2013). Consequently, each test was analyzed separately, and the results from these field tests will be discussed individually.
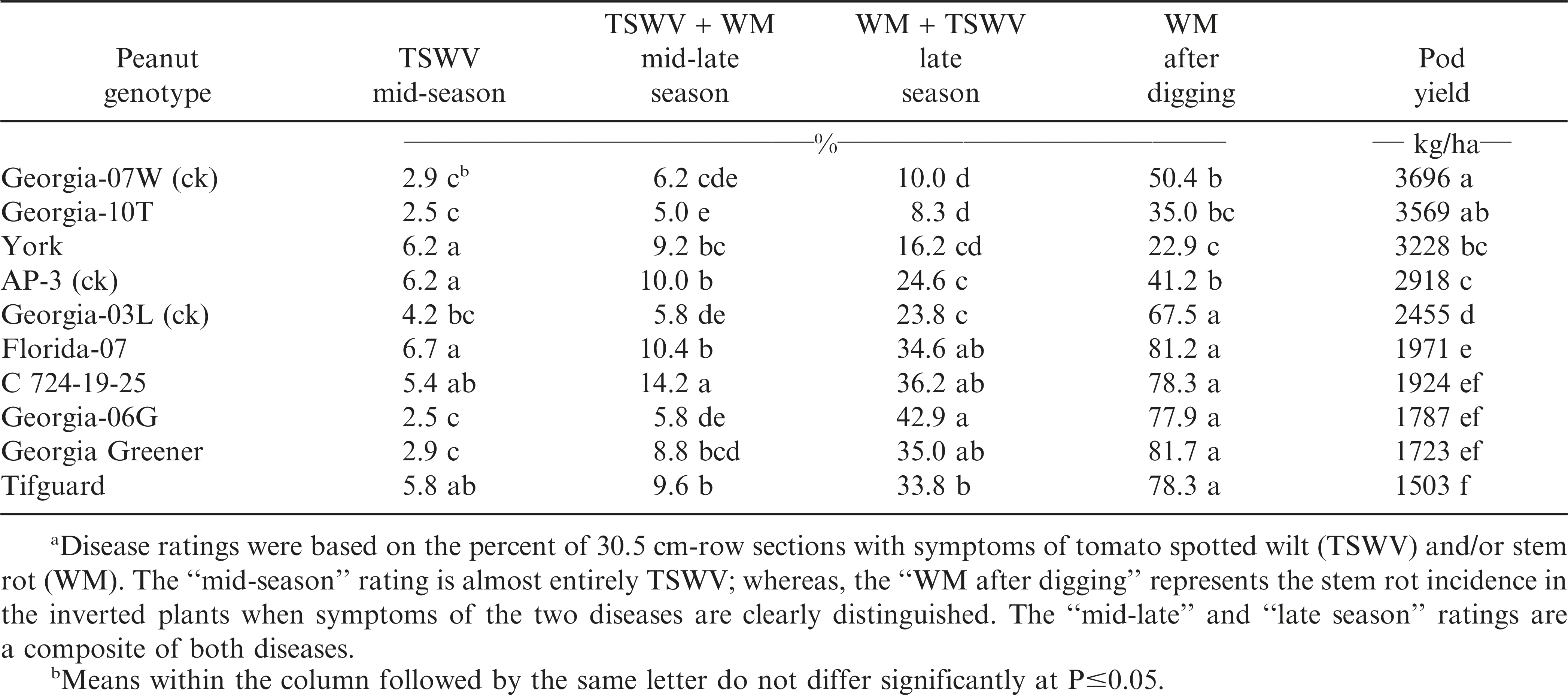
During 2010, all three check cultivars, Georgia-07W, AP-3, and Georgia-03L, were included (Table 1). TSWV incidence at mid-season and at mid-late season was the lowest among the Georgia cultivars, ‘Georgia-06G’ (Branch, 2007a), ‘Georgia-10T’ (Branch and Culbreath, 2011), Georgia-07W, ‘Georgia Greener’ (Branch 2007b), and Georgia-03L. However, stem rot (WM) results by late-season and after digging had Georgia-07W (ck), Georgia-10T, and ‘York’ (Gorbet and Tillman, 2011) with less disease incidence and higher pod yields that the other genotypes. It is also interesting to note that the currently grown runner-types, Florida-07, Georgia-06G, Georgia Greener, and ‘Tifguard’ (Holbrook et al., 2008), were consistently and equally susceptible to stem rot (WM) all four years (Tables 1-4).
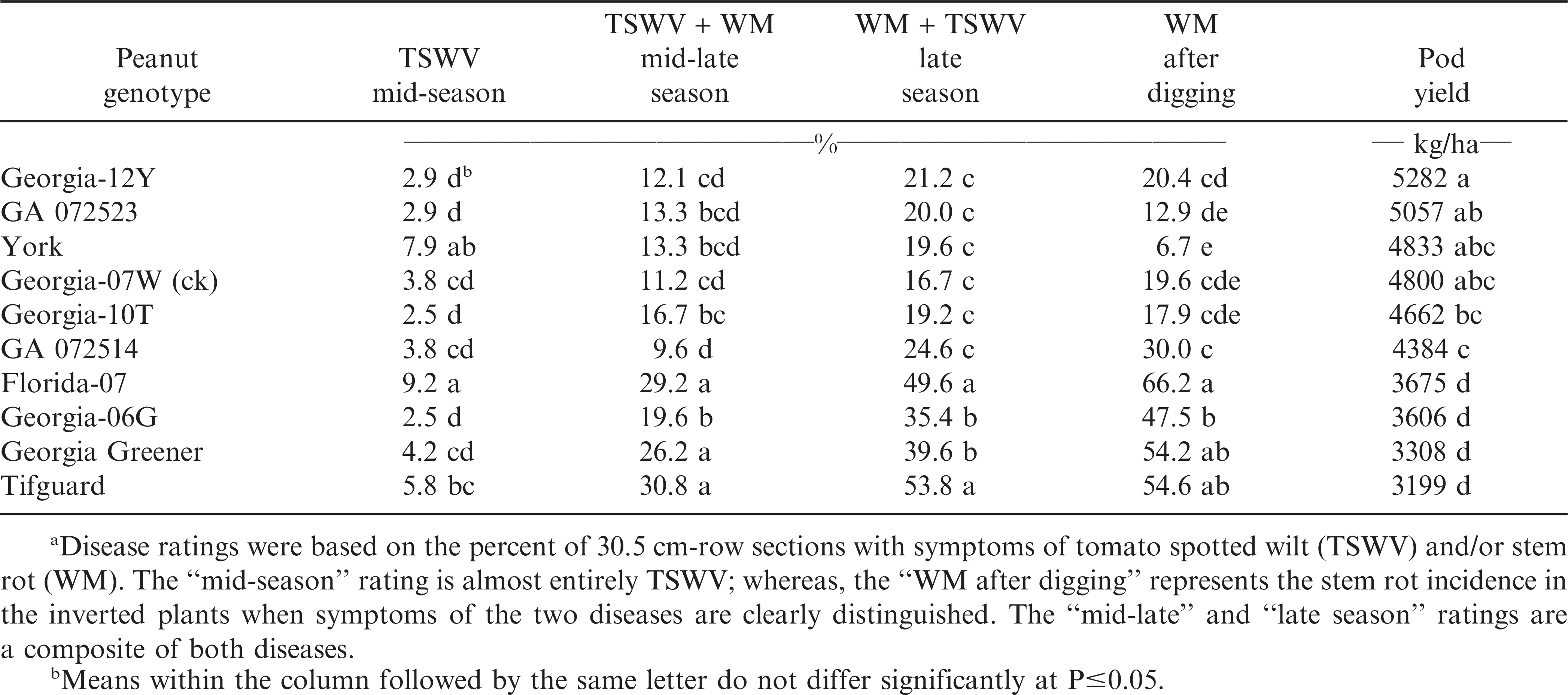
Beginning in 2011, Georgia-07W was the only check cultivar used since the other two were no longer grown (Table 2). At mid-season and mid-late season, TSWV was the least among Georgia-06G, Georgia-10T, GA 072514, GA 072523, ‘Georgia-12Y’ (Branch, 2013), and the check cultivar, Georgia-07W. However again by late-season and after digging, stem rot (WM) disease was the lowest and pod yield the highest among Georgia-12Y, York, GA 072523, Georgia-07W (ck), and Georgia-10T.
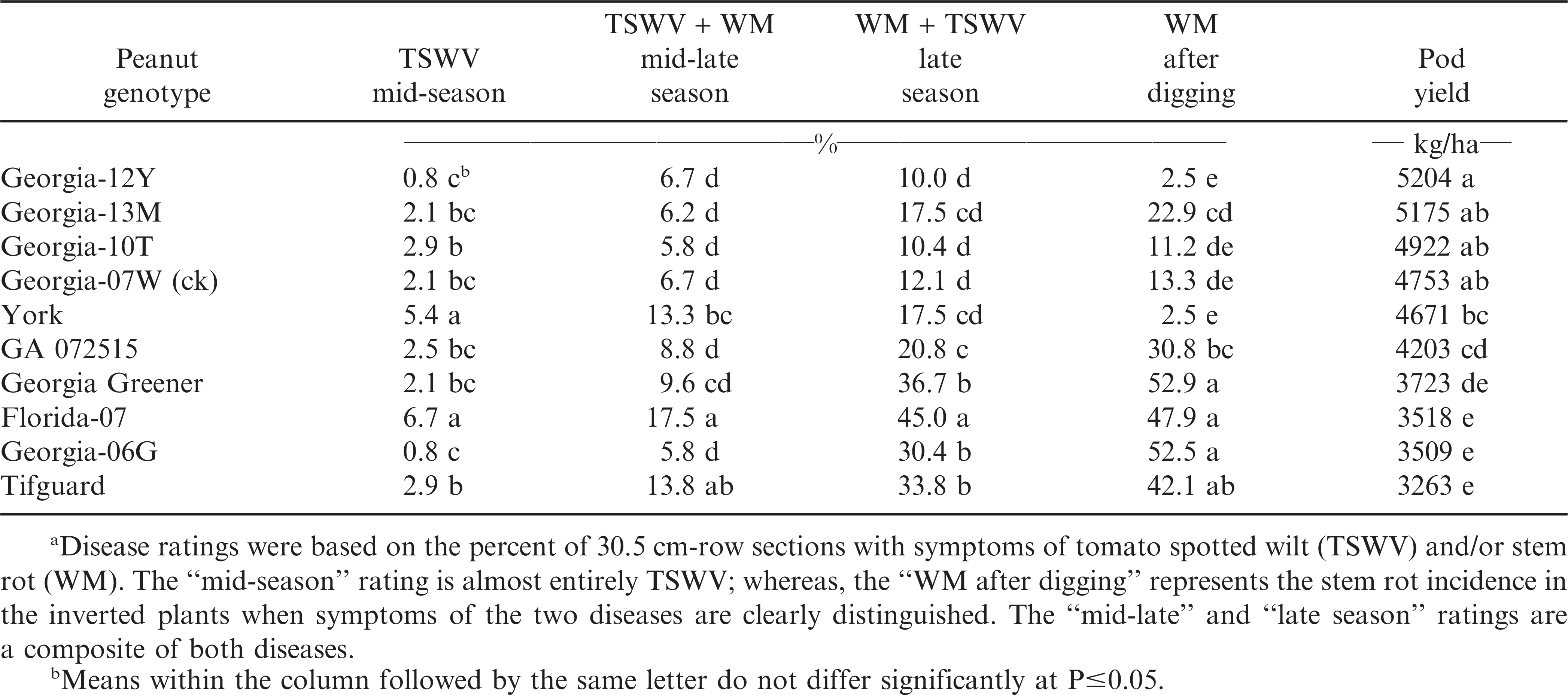
Likewise in 2012, TSWV at mid-season and TSWV and stem rot (WM) at mid-late season was the lowest with Georgia-06G, Georgia-12Y, ‘Georgia-13M’ (Branch, 2014), Georgia-10T, Georgia-07W (ck), GA 072505, and Georgia Greener (Table 3). However by late-season and after digging, stem rot (WM) disease incidence was the lowest and pod yield was the highest for Georgia-12Y, Georgia-13M, Georgia-10T, Georgia-07W (ck) and York.
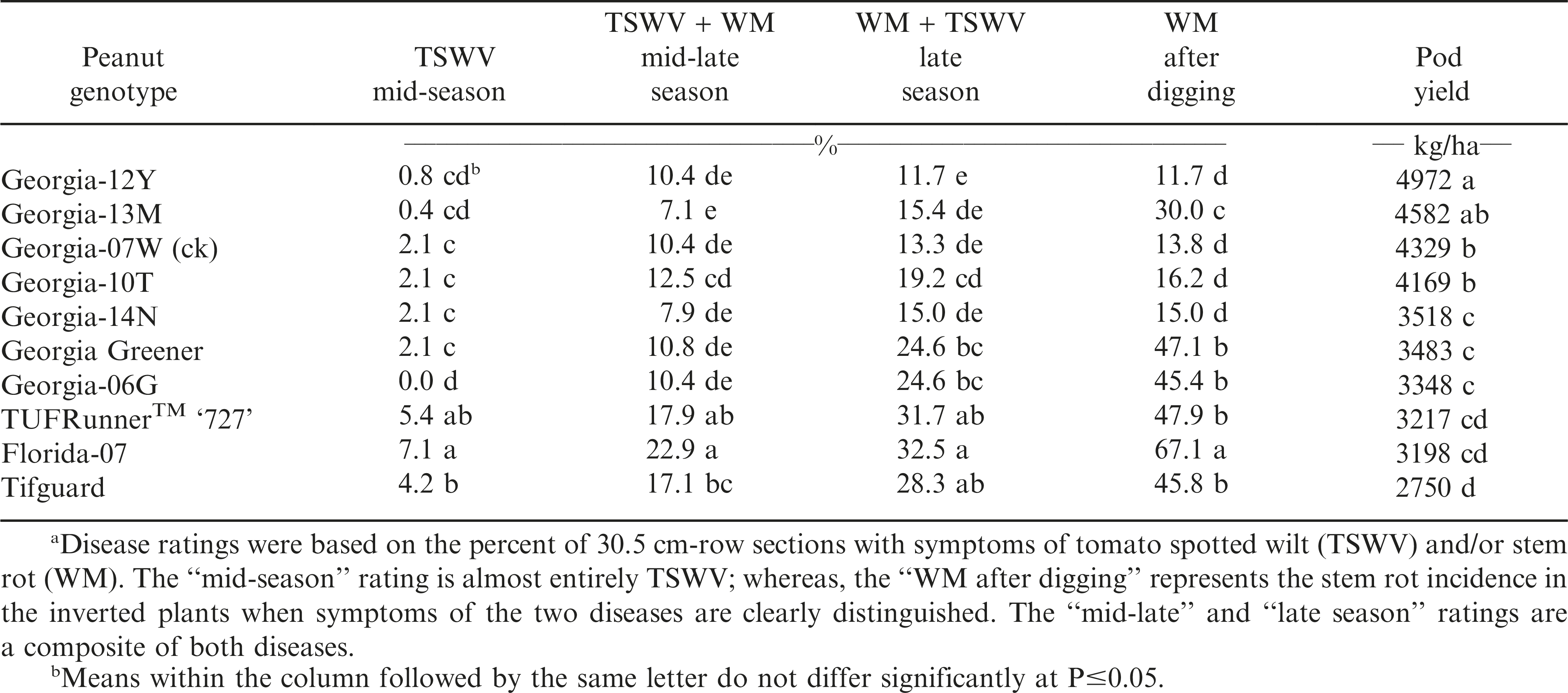
Again in 2013 (Table 4), TSWV disease incidence at mid-season and mid-late season was the lowest for Georgia-13M, Georgia-12Y, Georgia-07W (ck), Georgia-10T, ‘Georgia-14N’ (Branch and Brenneman, 2015), Georgia Greener, and Georgia-06G. Stem rot (WM) disease resistance at late-season and after digging was lowest and pod yield highest with Georgia-12Y, Georgia-13M, Georgia-07W (ck) and Georgia-10T. However, Georgia-14N had similarly low stem rot (WM) disease incidence, but pod yield was significantly lower than these other resistant cultivars; whereas, Georgia-13M had higher stem rot (WM) disease incidence after digging but higher pod yields.
Conclusions
The results from this 4-yr field evaluation study indicated that the greatest combination of TSWV and stem rot (white mold) disease resistance and highest consistent pod yield over years were in runner-type peanut cultivar Georgia-12Y, along with York, Georgia-07W, and Georgia-10T. Georgia-12Y, York, and Georgia-10T are later-maturing than the medium maturity check cultivar, Georgia-07W. Improvements have occurred, and will continue, in peanut breeding for resistance to two major disease problems, tomato spotted wilt and stem rot (white mold).
References
Branch W.D 1994 Registration of ‘Georgia Browne’ Crop Sci ., 34 : 1125 - 1126 .
Branch W.D 2004 Registration of ‘Georgia-03L’ peanut Crop Sci 44 : 1485 - 1486 .
Branch W.D 2007a Registration of ‘Georgia-06G’ peanut J. Plant Reg 1 : 120 .
Branch W.D 2007b Registration of ‘Georgia Greener’ peanut J. Plant Reg 1 : 121 .
Branch W.D 2012 Registration of ‘Georgia-12Y’ peanut J. Plant Reg 7 : 151 - 153 .
Branch W.D 2014 Registration of ‘Georgia-13M’ peanut J. Plant Reg 8 : 253 - 256 .
Branch W.D and Brenneman T.B 1993 White mold and rhizoctonia limb rot resistance among advanced Georgia peanut breeding lines Peanut Sci 20 , 124 – 126 .
Branch W.D and Brenneman T.B 2008 Registration of ‘Georgia-07W’ peanut J. Plant Reg 2 : 88 - 91 .
Branch W.D and Brenneman T.B 2009 Field evaluation for the combination of white mold and tomato spotted wilt disease resistance among peanut genotypes Crop Protection 28 : 595 - 598 .
Branch W.D and Brenneman T.B 2015 Registration of ‘Georgia-14N’ peanut J. Plant Reg 9 : 159 - 161 .
Branch W.D and Csinos A.S 1987 Evaluation of peanut cultivars for resistance to field infection by Sclerotium rolfsii Plant Disease 71 : 268 - 270 .
Branch W.D and Culbreath A.K 2011 Registration of ‘Georgia-10T’ peanut J. Plant Reg 5 : 279-281 .
Culbreath A Beasley J , Kemerait R , Prostko E. , Brenneman T. , Smith N. , Tubbs S. , Paz J. , Olatinuro R. , Tillman B. , Gevens A. , Weeks R. , Hagan A. 2009 Peanut Rx – The 2009 version of the peanut disease risk index . In: Prostko E. P (Ed.) 2009 Peanut Update, Univ. of Georgia, Coop. Ext. Serv CSS-09-0114 , pp. 41 - 56 .
Gorbet D.W 2007 Registration of ‘AP-3’ peanut J. Plant Reg 1 : 126 - 127 .
Gorbet D.W and Shokes F.M 2002 Registration of ‘C-99R’ peanut Crop Sci 42 : 2207 .
Gorbet D. W Tillman B. L 2009 Registration of ‘Florida-07’ peanut J. Plant Reg 3 : 14 - 18 .
Gorbet D.W and Tillman B.L 2011 Registration of ‘York’ peanut J. Plant Reg 5 : 289 - 294 .
Gorbet D.W Kucharek T.A Shokes F.M and Brenneman T.B 2004 Field evaluations of peanut germplasm for resistance to stem rot caused by Sclerotium rolfsii Peanut Sci 31 : 91 - 95 .
Gorbet D.W Norden A.J Shokes F.M and Knauft D.A 1987 Registration of ‘Southern Runner’ peanut Crop Sci 27 : 817 .
Holbrook C.C Timper P Culbreath A.K and Kvien C.K 2008 Registration of ‘Tifguard’ peanut J. Plant Reg 2 : 92 - 94 .
Kemerait R 2012 Peanut In: : Williams-Woodward J (Compl.) 2010 Georgia Plant Disease Loss Estimates, Univ. of Georgia, Coop. Ext. Serv. Ann. Publ 103-3 , p. 13 .
Kemerait R 2013 Peanut In : Williams-Woodward J. (Compl.) 2011 Georgia Plant Disease Loss Estimates, Univ. of Georgia, Coop. Ext. Serv. Ann. Publ 102-4 , p. 13 .
Kemerait R 2014 Peanut In : Williams-Woodward J. (Compl.) 2012 Georgia Plant Disease Loss Estimates, Univ. of Georgia, Coop. Ext. Serv. Ann. Publ. , p. 12 .
Tillman B.L Gorbet D.W and Anderson P.C 2007 Influence of planting date on yield and spotted wilt of runner market type peanut Peanut Sci 34 : 79 - 84 .
Williams J.E and Drexler J.S 1981 A non-destructive method for determining peanut pod maturity Peanut Sci 8 : 134 - 141 .
Notes
- Professor, University of Georgia, Dept. of Crop & Soil Sciences, Coastal Plain Experiment Station, 2360 Rainwater Road, Tifton, GA 31793-5766
- Professor, University of Georgia, Dept. of Plant Pathology, Coastal Plain Experiment Station, 2360 Rainwater Road, Tifton, GA 31793-5766 *Corresponding author's email: wdbranch@uga.edu
Author Affiliations