Introduction
Peanut (Arachis hypogaea L.), a leguminous crop, is capable of biological nitrogen fixation (BNF) by Bradyrhizobium (Schiffman and Alper, 1968; Shimshi et al., 1967; Walker et al., 1976). Inoculants can be applied with the seed or as granular or liquid products in the seed furrow at planting to ensure adequate amounts of Bradyrhizobium are present for root infection (Baughmann et al., 2007; Chapin et al., 2007; Godsey et al., 2007; Jordan, 2008a). Research indicates that in-furrow applications are often more effective than application to the seed when peanut is planted in fields that have never been seeded to peanut (Baughmann et al., 2007; Lanier et al., 2005). Cost of Bradyrhizobium inoculant is relatively low compared with other variable costs (Bullen and Jordan, 2008). Approximately 77% of growers in North Carolina apply inoculant to peanut (Rhodes et al., 2008).
Crop rotation is a critical component of pest management in peanut (Lamb et al., 1993; Rodriguez-Kabana et al., 1987; Rodriguez-Kabana and Touchton, 1984). Increasing the number of non-peanut crops between plantings of peanut often decreases incidence of disease and nematodes and can result in higher peanut yield (Bailey and Matyac, 1988; Pataky et al., 1983; Rodriquez-Kabana et al., 1987; Rodriguez-Kabana, 1984). Peanut also offer advantages for non-N fixing crops such as corn (Zea mays L.) and cotton (Gossypium hirsutum L.) by fixing N2 into approximately 124 kg/ha soil N when planted the year prior to these crops (Elkan, 1995).
Practitioners often pose the question of whether fumigation for Cylindrocladium black rot (caused by Cylindrocladium parasiticum) is needed if a certain number of non-peanut crops separate peanut plantings or if soybean [Glycine max (l.) Merr.], a host for Cylindrocladium black rot, is included in the rotation. Similarly, determining if the number of non-peanut crops between peanut plantings that result in a positive response to inoculation can be established would benefit practitioners. Although benefits of BNF to other non-legume crops in rotation with peanut are relatively well defined, interactions of crop rotation and inoculation are not well defined for peanut with respect to the role of rotation and rotation length on survival of Bradyrhizobium. Cooperative Extension Services in several states in the United States recommend inoculation of peanut regardless of rotation (Baughmann et al., 2007; Chapin et al., 2007; Godsey et al., 2007; Jordan, 2008a). A definite length of rotation when peanut will respond to inoculation has not been established for Virginia market type peanut. However, there is some debate among peanut growers and their advisors as to whether this approach is viable given the diversity factors that can influence nodulation of peanut by Bradyrhizobium. Therefore, research was conducted in North Carolina to determine the effect of crop rotation on peanut response to Bradyrhizobium.
Material and Methods
Experiments were conducted in North Carolina from 1999 through 2006 at the Border Belt Research Station near Whiteville, the Peanut Belt Research Station located near Lewiston-Woodville, and the Upper Coastal Plain Research Station near Rocky Mount on Norfolk sandy loam soil (fine-loamy, siliceous, thermic Aquic Paleudalts). Soil pH ranged from 5.7 to 6.1 and soil organic matter content ranged from 1.7 to 2.2%. The cultivars NC-V 11 (Wynne et al., 1991) (Lewiston-Woodville and Rocky Mount) and Perry (Isleib et al., 2003) (Whiteville) were planted in conventionally prepared seedbeds in early May to achieve a final in-row plant population of 13 plants/m. Plot size was 12 rows (Lewiston-Woodville and Whiteville) or 8 rows (Rocky Mount) by 15 m. Row spacing at all locations was 91 cm.
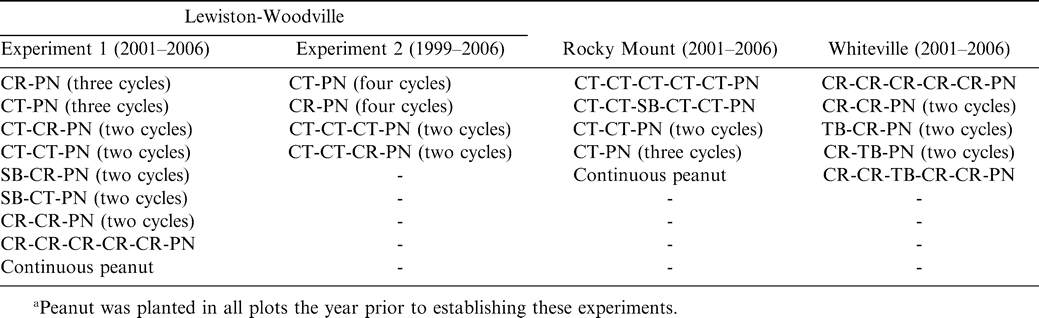
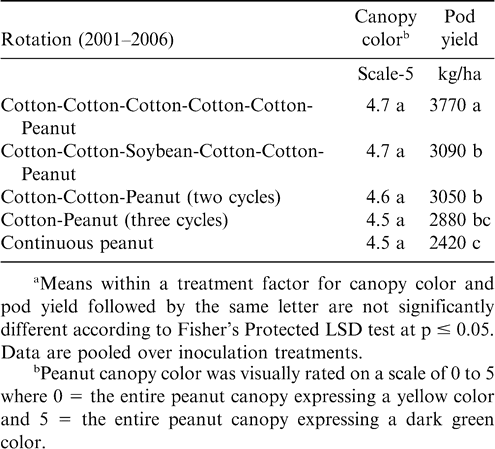
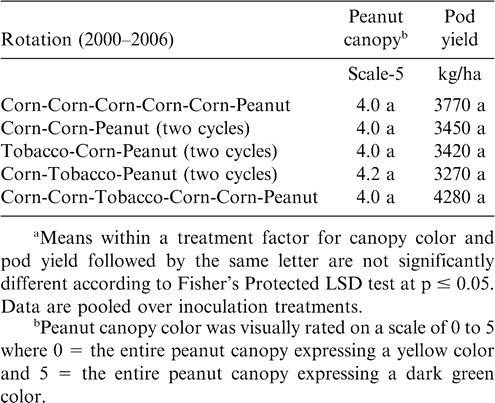
Cropping systems for the duration of the experiment varied considerably depending upon location (Table 1). At Lewiston-Woodville in one experiment, crop rotations from 2001–2006 included continuous peanut, peanut separated by five years of corn, three cycles of corn-peanut or cotton-peanut, and two cycles of corn-corn-peanut, cotton-cotton-peanut, cotton-corn-peanut, soybean-corn-peanut, or soybean-cotton-peanut. Peanut was planted in the entire test area during 2000. In a second experiment from 1999–2006 at this location, rotations included two cycles of cotton-cotton-cotton-peanut or cotton-cotton-corn-peanut and four cycles of corn-peanut or cotton-peanut. Peanut was planted in the entire test area during 1998. At Rocky Mount, rotations from 2001–2006 included five years of cotton followed by peanut, three cycles of cotton-peanut, two cycles of cotton-cotton-peanut, cotton-cotton-soybean-cotton-cotton-peanut, and continuous peanut. Rotations from 2001–2006 at Whiteville included five years of corn followed by peanut; two cycles of corn-corn-peanut, tobacco (Nicotiana tobacum L.)-corn-peanut, or corn-tobacco-peanut; and the rotation of corn-corn-tobacco-corn-corn-peanut.
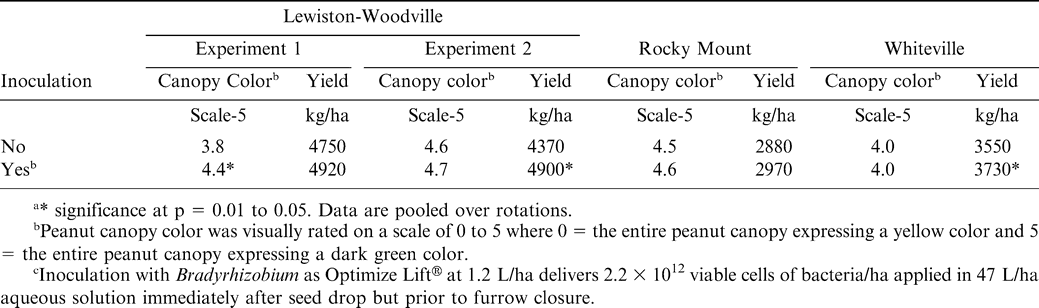
In 2006 at all locations, peanut was not inoculated or was inoculated with Bradyrhizobium (Optimize Lift®, EMD Crop Bioscience, Brookfield, WI) in the seed furrow at planting. Optimize Lift® delivers 2.2 × 1012 viable cells of bacteria/ha when applied at 1.2 L/ha in 47 L/ha aqueous solution immediately after seed drop but prior to furrow closure. Spray solution directly contacted seed. Peanut was not inoculated during any year of the experiment except in 2006. Peanut was planted in all fields the year prior to initiating the experiments. No attempt was made to quantify N concentration in soil.
Aldicarb [2-methyl-2-(methylthio)propionaldehyde O-methylcarbamoloxime] at 1.1 kg ai/ha was applied in the seed furrow. All other production and pest management practices were held constant over the entire test area and were based on Cooperative Extension Service recommendations appropriate for the region (Brandenburg, 2007; Jordan, 2008a 2008b; Shew, 2007).
During the second week of August in 2006, color of the entire peanut canopy was estimated visually on a scale of 0 to 5 where 0 = the entire peanut canopy expressing a pale yellow color and 5 = the entire peanut canopy expressing a dark green color. Determining canopy color at this stage of peanut development minimized possible confounding of canopy color due to development of Cylindrocladium black rot and other diseases resulting from crop rotation. While determining N concentration in peanut leaves or recording chlorophyll content would have been more informative than assessing the canopy visually, practitioners most likely will use a visual assessment for this comparison. Peanut was dug and vines inverted in late September or early October based on pod mesocarp color determination (Williams and Drexler, 1981). Digging was initiated when approximately 65% of pods were in the brown and black color pod mesocarp color category (Jordan et al., 2005). Pods were harvested 4 to 7 d after digging and vine inversion.
The experimental design in all experiments was a split plot with sub-plots replicated four times. Cropping system served as the whole plot unit and inoculation treatment served as the split plot unit. Data for canopy color and pod yield were subjected to analysis of variance appropriate for the treatment structure (SAS, 2006). Means of significant main effects and interactions were separated using Fisher's Protected LSD test at p ≤ 0.05.
Results and Discussion
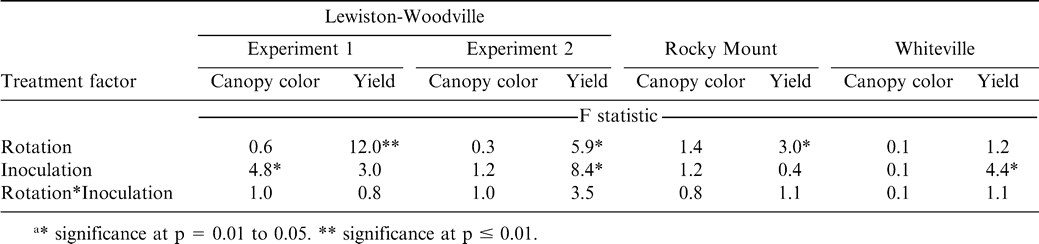
The interaction of rotation by inoculation was not significant for peanut canopy color or peanut pod yield in any of the experiments or locations (Table 2). Crop rotation did not affect peanut canopy color but did affect pod yield in three of the four experiments (Table 2). Inoculation affected canopy color in one experiment at Lewiston-Woodville and affected pod yield in two of four experiments (Table 2). Data for peanut canopy color and pod yield are presented for all experiments regardless of significance of the main affect at a location or within an experiment at a particular location.
Crop rotation
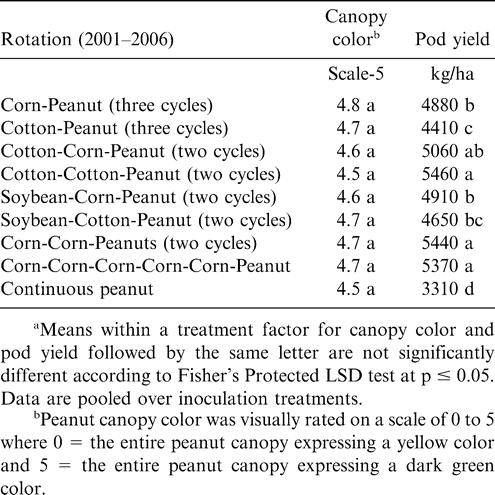
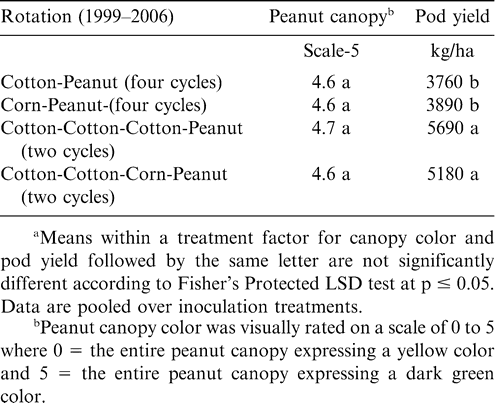
Increasing the number of years between peanut plantings did not affect canopy color but did affect pod yield at Lewiston-Woodville in both experiments (Tables 3 and 4). The lowest pod yield in the first experiment was noted for continuous peanut while the highest pod yields were noted for two cycles of corn-corn-peanut, cotton-cotton-peanut, or cotton-corn-peanut and the rotation with five years of corn and one year of peanut (Table 3). Rotation with only one year of corn or cotton between peanut or including soybean in the rotation (two cycles of soybean-corn-peanut or soybean-cotton-peanut) yielded more than continuous peanut but lower than peanut in several of the highest yielding rotations (Table 3). In the second experiment at Lewiston-Woodville, peanut canopy color was not affected by rotation but yield was higher with two cycles of cotton-cotton-cotton-peanut or cotton-cotton-corn-peanut compared with three cycles of corn-peanut or cotton-peanut (Table 4).
At Rocky Mount, pod yield but not canopy color was affected by rotation (Table 5). The highest yield was noted when five years of cotton separated peanut plantings (Table 5). Yield was similar for rotations including two cycles of cotton-cotton-peanut, cotton-cotton-soybean-cotton-cotton-peanut, and three cycles of cotton-peanut, and yield of peanut from these rotations was lower than yield of peanut following five years of cotton. Yield following a 3-yr cycle of cotton-peanut and continuous peanut was similar. In contrast, canopy color and pod yield at Whiteville was not affected by rotation (Table 6). However, at this location alternating peanut with other crops and continuous peanut was not included. Cylindrocladium black rot was present at all locations, and lack of a response to rotation at Whiteville may have been associated with planting the Cylindrocladium black rot resistant cultivar Perry (Shew, 2007) as well as rotations at this location being at least three years in all cases. Increasing the length of rotation increased yield compared with shorter rotations when the Cylindrocladium black rot susceptible cultivar NC-V 11 was planted at the other locations (Shew, 2007).
Peanut response to crop rotation observed in these experiments is similar to previous findings demonstrating that increasing the number of non-peanut crops in the rotation generally increases peanut yield (Bailey and Matyac, 1988; Lamb et al., 1993; Pataky et al., 1983). These data also demonstrate that planting at least two years of corn or cotton or a sequence of these crops often optimizes yield. Results from these experiments also support previous findings demonstrating that corn and cotton are more effective than soybean in maintaining peanut yield (Jordan et al., 2002). Variation in canopy color when comparing experiments could have been a result of the time of evaluation relative to planting, infection by Bradyrhizobium, and soil N remaining from the previous crop. Recording canopy color multiple times during the growing season would have been more informative.
Inoculation with Bradyrhizobium
Inoculation affected peanut canopy color in one experiment at Lewiston-Woodville but not in the second experiment at this location or in experiments at Rocky Mount or Whiteville (Table 7). Pod yield was not affected by inoculation in Experiment 1 at Lewiston-Woodville but did increase by 530 kg/ha in the second experiment (Table 7). Inoculation did not affect pod yield at Rocky Mount (Table 7). However, inoculation with Bradyrhizobium increased yield at Whiteville by 180 kg/ha (Table 7).
Differences in peanut response to rotation and inoculation varied in these experiments, but the interaction of rotation by inoculation was not significant in any of the experiments. The objective of this research was to determine the relationship between the number of non-peanut crops between peanut plantings and peanut response to in-furrow application of Bradyrhizobium. In these experiments, rotations consisted of as many as five years of corn or cotton between peanut plantings as well as continuous peanut. Results from these experiments indicate that using the number of crops between peanut plantings or visually comparing canopy color does not define whether or not a positive yield response to in-furrow inoculation with Bradyrhizobium will occur. Peoples et al. (1992) also reported no interaction between crop rotations and inoculant treatment.
Limitations in extrapolating results from this experiment to other regions of the US peanut belt and to other years and differing environments exist. For example, response to inoculation can vary due to environmental conditions and possibly other factors specific to the year when inoculation treatments were compared. It is possible that conditions during 2006 may have had a major impact on peanut response to inoculation, possibly as much as previous cropping system. Additional research is needed to evaluate possible interactions of crop rotation and inoculation on a wider range of environmental and edaphic conditions in additional geographical regions of peanut production. However, expense of inoculation is $12/ha or less depending upon product selection, and this constitutes approximately 1% or less of production cost of $1971/ha to produce Virginia market type peanut (Bullen and Jordan, 2008). Therefore, inoculation of peanut regardless of length of rotation between peanut plantings will often pay dividends and will not be costly in years that yield increases do not occur.
Acknowledgements
This research was supported financially by the North Carolina Peanut Growers Association, Inc. Carl Murphey and Brenda Penny and staff at the Border Belt Tobacco Research Station, Peanut Belt Research Station, and the Upper Coastal Plain Research Station provided technical support.
Literature Cited
Bailey J. E. and Matyac C. A. 1988 A decision model for use of fumigation and resistance to control Cylindrocladium black rot of peanuts. Plant Disease 73 4 : 323 – 326 .
Baughman T. , Dotray P. , Grichar J. , Black M. , Woodward J. , Trostle C. , Russell S. , Crumley C. , Porter P. , New L. , Baumann P. , and McFarland M. 2007 Texas peanut production guide Texas Cooperative Extension Ser College Station, TX [http://peanut.tamu.edu/pdfs/productionguide07.pdf] accessed 02/22/2008. 58.
Brandenburg R. L. 2007 Peanut insect management. 75 – 93 In 2007 Peanut Information North Carolina Coop. Ext. Ser. AG-331, 132 .
Bullen S. G. and Jordan D. L. 2008 Peanut production budgets. 6 – 15 In 2008 Peanut Information North Carolina Cooperative Extension Service Publication AG-331, 134.
Chapin J. W. , Prostko E. P. , and Thomas J. S. 2007 Peanut money-maker production guide – 2007. Clemson University Cooperative Extension Ser., Clemson, SC. Circular 588. [http://virtual.clemson.edu/groups/peanuts/mmaker06.pdf] accessed: 02/22/2008. 54.
Elkan G. H. 1995 Biological nitrogen fixation in peanuts. 286 – 300 In Pattee H. E. and Stalker H. T. Advances in Peanut Science American Peanut Research and Education Soc Stillwater, OK 614 .
Godsey C. , Damicone J. , Mulder P. , Medlin C. , Kizer M. , Noyes R. , and Zhang H. 2007 2007 peanut production guide for Oklahoma. Oklahoma Cooperative Extension Ser. Circular E-806. Oklahoma Cooperative Extension Service, Stillwater, OK. [http://www.peanut.okstate.edu/prodinfo/E-806-2007.pdf] accessed: 02/22/2008. 88 .
Isleib T. G. , Rice P. W. , Mozingo R. W. , Bailey J. E. , Mozingo R. W. , and Pattee H. E. 2003 Registration of ‘Perry’ peanut. Crop Sci 43 : 739 – 740 .
Jordan D. L. 2008a Peanut production practices. 21 – 44 In 2008 Peanut Informatio, North Carolina Cooperative Extension Service Publication AG-331, 134.
Jordan D. L.
2008b
Peanut weed management.
45
–
72
In
2008 Peanut Information,
North Carolina Cooperative Extension Service Publication AG-331,
134.
Jordan D. , Johnson D. , Spears J. , Penny B. , Shew B. , Brandenburg R. , Faircloth J. , Phipps P. , Herbert A. , Coker D. , and Chapin J. 2005 Determining peanut pod maturity and estimating the optimal digging date: using pod mesocarp color for digging Virginia market type peanut. North Carolina Cooperative Extension Service Publication AG-633.
Jordan D. L. , Bailey J. E. , Barnes J. S. , Bogle C. R. , Bullen S. G. , Brown A. B. , Edmisten K. L., Dunphy E. J., and Johnson P. D. 2002 Yield and economic return of ten peanut-based cropping systems. Agron. J 94 : 1289 – 1294.
Lamb M. C. , Davidson J. I. , and Butts C. L. 1993 Peanut yield decline in the Southeast and economically reasonable solutions. Peanut Sci 20 : 36 – 40 .
Lanier J. E. , Jordan D. L. , Spears J. F. , Wells R. , and Johnson P. D. 2005 Peanut response to inoculation and nitrogen fertilizer. Agron. J 97 : 79 – 84 .
Pataky J. K. , Beute M. K. , Wynne J. C. , and Carlson G. A. 1983 A critical-point yield loss model for Cylindrocladium black rot of peanut. Phytopathol 73 : 1559 – 1563 .
Peoples M. B. , Bell M. J. , and Bushby H. V. A. 1992 Effect of rotation and inoculation with Bradyrhizobium on nitrogen fixation and peanut yield. Australian J. Agric. Res 43 : 595 – 607.
Rhodes R. , Smith L. , Williams M. , Smith P. , Winslow F. , Cochran A. , Simonds B. , Whitehead A. , Ellison C. , Pearce J. , Tyson C. , Uzzell S. , Harrelson R. , Fountain C. , Shaw M. , Bridgers T. , Jordan D. L. , Brandenburg R. L. , and Shew B. B. 2008 Summary of production and pest management practices by top growers in North Carolina. Proc. Am. Peanut Res. and Ed. Soc 40 . (in press).
Rodriguez-Kabana R. , Ivey H. , and Backman P. A. 1987 Peanut-cotton rotations for management of Meloidogyne arenaria. J. Nematol 19 : 484 – 487 .
Rodriguez-Kabana R. and Touchton J. T. 1984 Corn and sorghum rotations for management of Meloidogyne arenaria in peanut. Nematropica 14 : 26 – 36 .
SAS Institute 2006 GLM Procedure. 100 SAS Campus Drive, Cary, NC 27513-2414.
Schiffman J. and Alper Y. 1968 Effects of Rhizobium – inoculum placement on peanut inoculation. Exp. Agric 4 : 203 – 208 .
Shew B. B. 2007 Peanut disease management. 94 – 118 In 2007 Peanut Information, North Carolina Coop. Ext. Ser. AG-331, 132.
Shimshi D. , Schiffman J. , Kost Y. , Bielorai H. , and Alper Y. 1967 Effects of soil moisture regime on nodulation of inoculated peanuts. Agron. J 59 : 397 – 400 .
Walker M. E. , Minton N. A. , and Dowler C. C. 1976 Effects of herbicides, a nematicide and Rhizobium inoculant on yield, chemical composition and nodulation of Starr peanuts (Arachis hypogaea L.). Peanut Sci 3 : 49 – 51 .
Williams E. J. and Drexler J. S. 1981 A non-destructive method for determining peanut pod maturity. Peanut Sci 8 : 134 – 141 .
Wynne J. C. , Coffelt T. A. , Mozingo R. W. , and Anderson W. F. 1991 Registration of ‘NC-V 11’ peanut. Crop Sci 31 : 484 – 485 .
Notes
Author Affiliations
1 Professor and Research Technician, Department of Crop Science, North Carolina State Univ., Box 7620, Raleigh, NC 27695-7620.
2 Former and current Superintendents, Peanut Belt Research Station, 112 Research Station Lane, Lewiston-Woodville, NC 27849-9552.
3 Superintendent, Upper Coastal Plain Research Station, 2811 Nobles Mill Pond Road, North Carolina Department of Agriculture and Consumer Services, Rocky Mount, NC 27801-9276.
4 Border Belt Tobacco Research Station, 86 Border Belt Drive, North Carolina Department of Agriculture and Consumer Services, Whiteville, NC 28472-6828.
*Corresponding author: david_jordan@ncsu.edu.