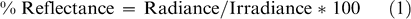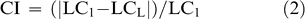Introduction
The accurate assessment of peanut (Arachis hypogaea L.) maturity is one of the most important economic decisions a grower must make. Digging too early can cause loss of yield and grade (the two characteristics that determine the economic return to the grower) and lower flavor quality through the incomplete conversion of sugar into oil as the peanut seed develops (Fincher et al., 1980). However, digging an overly mature crop often leads to mechanical losses, either through the retention of pods in the soil during the digging process or the separation of pods during the picking process, both related to deterioration of the peg as it ages. Digging losses are typically 8% of the total crop yield, but can reach 40% at dates beyond optimal maturity (Young et al., 1982; Lamb et al., 2004).
Several methods exist for determining peanut maturity including: days after planting; Langleys Index; internal hull color (Shellout Method); oil color; methanolic extraction; kernel density; seed/hull ratio (SHMI); arginine maturity index (AMI); physiological maturity index; and the hull scrape method (Sanders et al., 1982a,1982b). However, the most accepted and widely used method is the use of the “maturity profile board” based on the work of Williams and Drexler (1981). This method requires the removal of the exocarp from the peanut hull and classification of the shade of the mesocarp into specific color classes (white, yellow 1, yellow 2, orange, brown, and black), each with individual columns that represent successive stages of development within these color classes. This classification enables a grower to estimate the projection of optimal harvest date. While the Williams and Drexler (1981) method is the accepted industry standard, its accuracy is somewhat hampered due to the subjective assessment of color classes, the time consuming nature of the process, and the possible inapplicability of the color classes to current cultivars in production today.
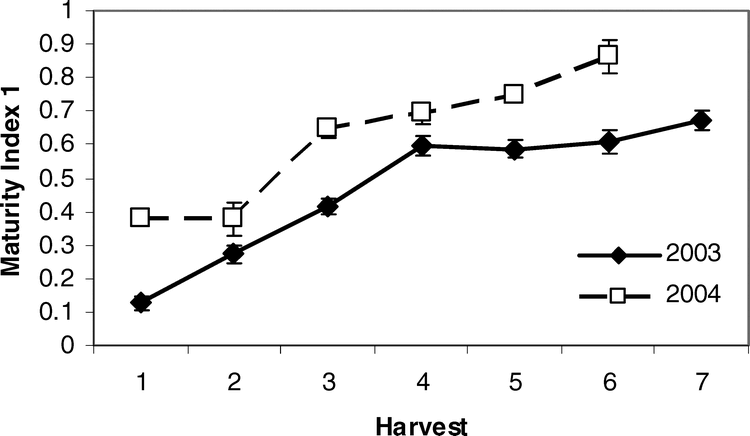
Rowland et al. (2006) demonstrated that classification into fine color classes may not be necessary to determine the maturity level of the crop. A maturity index, termed Maturity Index 1, was determined to be the best predictor of yield and grade, and thus economic maturity of the crop. Maturity index 1 was calculated as the sum of all pods classified within the three columns of the “brown” and within the six columns of the “black” divided by the total number of pods in all columns on the maturity profile board. Further, an alternative method of maturity assessment was developed for peanuts by testing and modifying existing degree day models that have been advocated for peanut but rarely utilized (Rowland et al., 2006). Degree day models have been used very successfully in other crops to determine optimal harvest timing including corn (Zea mays L.), soybean (Glycine max L.), cotton (Gossypium hirsutum L.), vegetables (NeSmith and Hoogenboom, 1994; Dufault, 1997; Perry et al., 1997; Andrade et al., 2000; Cober et al., 2001; Viator et al., 2005), and many non-crop species (Spano et al., 1999), but tested methods for current peanut cultivars are scarce. Rowland et al. (2006) determined that the degree day model first presented by Mills (1964) and modified by the addition of seasonal cumulative water received by the crop (via irrigation and rainfall) showed an adjusted R2 value of 0.93 with crop maturity. Therefore, Rowland et al. (2006) illustrated that a modified degree day method could provide an objective, accurate, and simple assessment of peanut maturity as an alternative to the more subjective and time consuming maturity profile board.
Optimally, the combination of degree day calculations with additional alternative maturity assessments could provide a wider choice for growers and may serve as validation for available degree day models. Further, to be the most beneficial, a maturity assessment method should be able to be applied relatively easily in a production setting, preferably without destructive harvesting of pod samples. The ideal methods would utilize measures of aboveground characteristics in the peanut plant that could be easily sampled and analyzed, or under the best case scenario, would be remotely sensed. Few studies have investigated the utility of remotely sensed plant characteristics for peanut, thus baseline information is needed to correlate with ground based data (Sanders et al., 2002; Sullivan and Holbrook, 2007). This study sought to test and evaluate canopy traits that may be correlated with crop maturity. Specifically, the objective of this study was to determine if plant canopy characteristics could be used successfully to predict peanut maturity, through the correlation of solute concentrations, chlorophyll content, nutrient analysis, and reflectance.
Materials and Methods
Planting and Crop Maintenance
Peanut (cv. Georgia Green) was planted in 2003 and 2004 at two research sites: one located in Dawson, GA and the other in Sasser, GA. The soil at Dawson was a Greenville fine sandy clay loam (fine, kaolinitic, thermic, Rhodic Kandiudults) while the soil at Sasser was a Tifton loamy sand (fine-loamy, kaolinitic, thermic, Plinthic, Kandiudults). At both sites, peanuts were sown in twin rows consisting of two planted rows (“twins”) 23 cm apart with a distance of 91 cm between the two outside rows of each set of twin rows. Inter-seed distance was 10 cm within each of the rows comprising the twin row. Both locations were subjected to management for optimum yield with respect to pest and weed management, and fertility.
The Dawson site was not irrigated in 2003 but was irrigated in 2004 using an overhead lateral sprinkler irrigation system with scheduled irrigation based on a modified Jensen-Haise potential evapotranspiration (ETo) calculation using atmospheric conditions (Jensen and Haise, 1963). Estimated ETo was multiplied by the crop coefficient for peanut (Harrison and Tyson, 1993) to estimate actual evapotranspiration (ETa). If rainfall was greater than ETa no irrigation was applied; ETa amounts were added up over a 3–5 day schedule and subsequently applied using the lateral irrigation system. The Sasser site was irrigated in both 2003 and 2004 using subsurface drip irrigation (Toro Ag, Aqua-Traxx, Bloomington, MN, USA3) installed 30 cm below the soil surface at a lateral spacing of 0.91m and with emitters spaced 30 cm apart. Individual emitter flow rate was maintained at 70 kPa at a rate of 1.0 liter per hour. Irrigation was scheduled with the same modified Jensen-Haise ETo equation employed at the Dawson site but utilizing the atmospheric parameters measured at the Sasser site. ETa amounts were replaced on a daily basis.
Harvest and Canopy Analyses
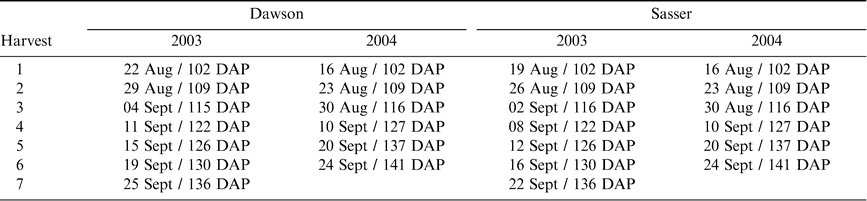
Test rows were harvested sequentially in 2003 and 2004 on a weekly basis and more frequently harvested as maturity progressed at each site (Table 1). At both sites, test rows consisted of 2 paired twin rows that were dug and inverted with a two row peanut inverter (Kelly Manufacturing Co., Inc., Tifton, GA, USA). These harvested rows were separated into three equal replicate sections (12 m each at Sasser and 9 m each at Dawson) and five plants were randomly collected from each section for maturity determination. One plant was chosen for leaf level analyses and one tetrafoliate leaf located on the main stem at the second nodal position was collected for the following measurements: SPAD chlorophyll content (Soil-Plant Analyses Development Unit, Minolta Corp., Ramsey, N.J., U.S.A.), Brix refractive index (Extech Instruments Corp./Spectrum Technologies, Inc., Plainfield, I.L., U.S.A.), and osmolality (Wescor, Inc., Logan, U.T., U.S.A.). The SPAD chlorophyll meter measures absorbance by plant tissues of wavelengths in the visible spectrum and serves as a measure of the relative internal concentration of chlorophylls a and b. One SPAD chlorophyll reading was taken on each of the four leaflets, avoiding the midrib, and then averaged for one chlorophyll reading per plant to correct for possible non-homogeneous distribution of chlorophyll throughout the leaf (Monje and Bugbee, 1992). Brix refractive index and percentage were measured by pre-freezing the four leaflets measured with the SPAD meter and pressing leaves in a syringe to extract sap. Osmolality was measured using a similar extraction method but using the third nodal position leaf on the same plants measured with the SPAD and Brix meter. In addition, fifteen to twenty second nodal apex leaves were collected from individual plants within each rep and pooled for analysis of percent N, P, K, Mg, Ca, S, and ppm of B, Zn, Mn, Fe, and Cu (Waters Laboratory, Camilla, GA).
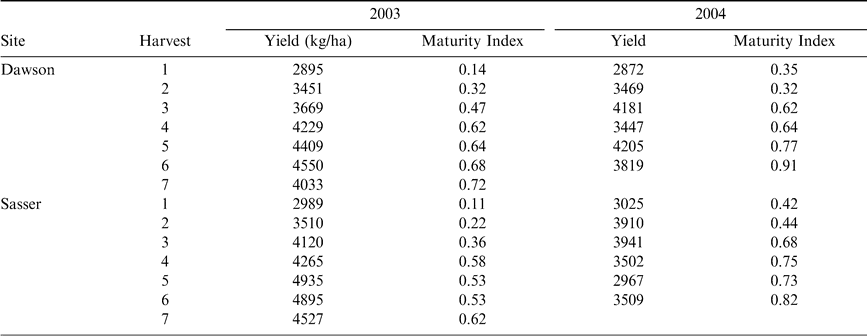
The plants were returned to the laboratory and a sample of approximately 150–200 pods was removed. The exocarp was removed through the use of high pressure washing in the presence of glass beads in 2003, and through pressure washing using a rotating turbo nozzle in 2004. Blasted pods were placed on maturity boards (Williams and Drexler, 1981) and final color classes determined by a single observer. Because it was previously determined that the total number of pods classed as brown and black divided by the total number of pods placed on the maturity profile board (Maturity Index 1, Table 2) was the best predictor of overall kernel maturity and grade (Rowland et al., 2006), this index was used as the response variable in all predictive models attempting to determine the correlation between leaf characteristics and peanut maturity.
The rest of each field section (containing inverted peanuts) was allowed to dry in the windrow for 2–3 days. Pods were then removed from the plants using a hand thresher (Kingaroy Engineering Works, Kingaroy, Australia). Pods were dried to between 7 and 10% moisture using either ambient or heated air flow in a 0.03 m3 dryer prior to the determination of field weight. Farmer stock grade (percent sound mature kernels) was determined using the procedures described by USDA (2006). Subsequent yields were calculated by taking field weight and subtracting the weight of loose shelled kernels and foreign material, and correcting for moisture levels in excess of 7 percent (Table 2).
Remote Sensing
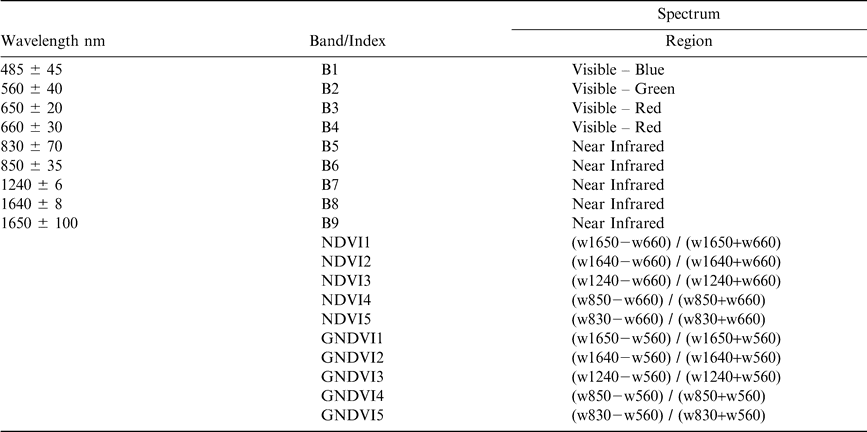
In 2004, reflectance measurements were collected using a hand-held CropScan Multispectral Radiometer (CropScan Inc, Minnesota). The CropScan utilizes narrow band interference filters to select discrete bands in the VIS and NIR regions of the electromagnetic spectrum. Nine bands were measured in this study within the 485–1,650 nm range (Table 3). The CropScan is equipped with upward and downward looking sensors in each band, and simultaneously acquires irradiance as well as radiance over the target. It is assumed that irradiance over the sensor head is equal to irradiance over the target. Radiance and irradiance were measured in millivolts, adjusted for temperature of the CropScan, and converted to an energy term. Percent reflectance was determined using the following equation:
All plot data were collected as close to solar noon as possible, under clear conditions. Data were collected at nadir, over row middles, from a height of 2 m to approximate a 1m2 spatial resolution on the ground. Remotely sensed data were acquired twice during the 2004 growing season: 23 August and 23 September, or 109 and 140 DAP, respectively.
Vegetation indices designed to reduce the impact of atmospheric attenuation, illumination, and bare soil contributions were calculated and used to evaluate changes in spectral response as the peanut crop neared maturity. Indices included two commonly used vegetation indices: the greenness normalized difference index (GNDVI) (Gitelson et al., 1996) and the normalized difference vegetation index (NDVI) (Rouse et al., 1974). Because the CropScan encompassed multiple bandwidths within the NIR and red, a series of NDVI and GNDVI indices were calculated and evaluated (Table 3).
Data Analysis
Factorial analysis of variance (ANOVA) determined differences in leaf traits in 2003 and 2004 between harvest dates and sites (SAS, 1997). Tukey's HSD multiple comparisons test (at p-value < 0.05) was used to separate means when significant differences existed among harvests. Pearson correlation analyses were performed examining the relationship between leaf characteristics and Maturity Index 1. Stepwise regression analysis using all measured leaf traits was performed to determine those traits that contributed most to the sample variance (e.g. that were the best predictors of Maturity Index 1). To calculate the percent change in the leaf characteristics explaining the greatest amount of variability in Maturity Index 1, the following Change Index was calculated:
where LC1 is the value of the leaf character at the first harvest, LCL is the value of the leaf character at the last harvest (harvest 7 in 2003 and harvest 6 in 2004). The CI values were plotted against harvest, fit with parabolic functions, and the R2 value assessed.
The remote sensing data were analyzed using SAS (1997). Because significant differences were observed between sites, each site was analyzed separately. Analysis of variance was used to evaluate differences in spectral bands and vegetation indices between harvests.
Results
Effects of Site and Harvest
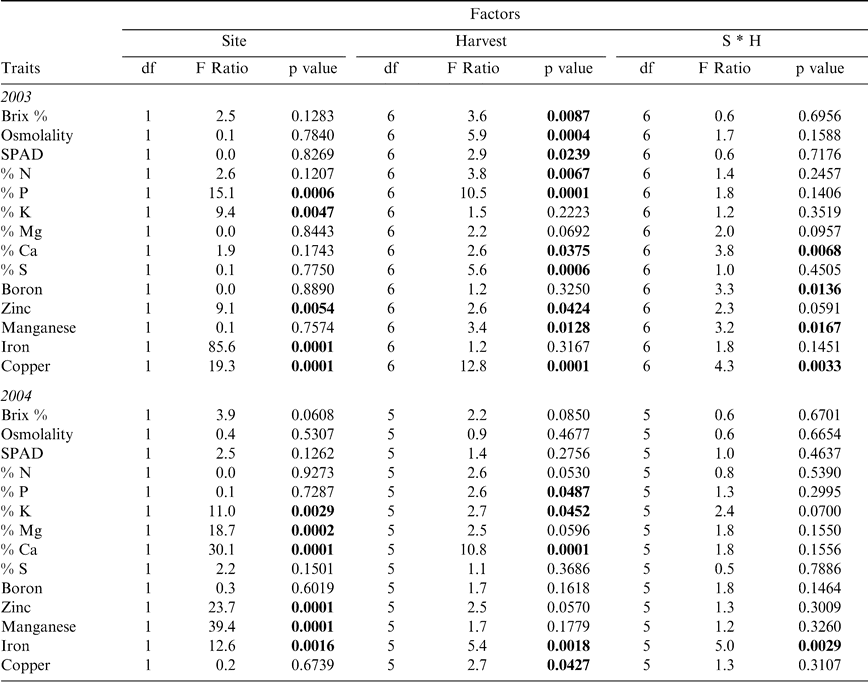
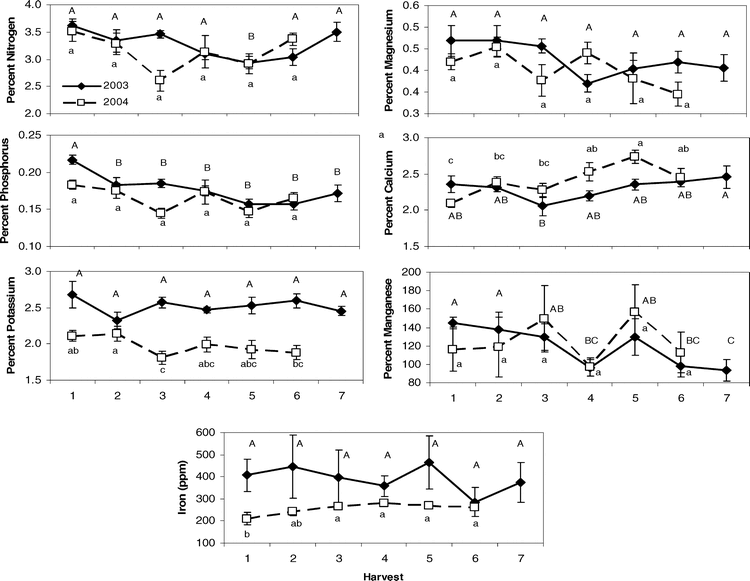
Canopy characteristics showed differences both between the two sites, Sasser and Dawson, and among the sequential harvests through the season as expected due to the progressive maturity of the crop across time (Table 2). Sites differed primarily in the potassium, zinc, and iron content of the crop in both years (Table 4), where the Dawson site had elevated levels of these nutrients in both 2003 and 2004. Despite the expectation that most leaf nutrients would show changes across the sampling period due to the high nutrient burden associated with physiologically maturing the crop and the oncoming senescence of the crop in general, many leaf characteristics showed no significant differences among harvests (Table 4). Magnesium and boron remained at consistent levels in leaf tissue over time in both 2003 and 2004; while potassium and iron showed no changes in 2003, and Brix, osmolality, SPAD, nitrogen, sulfur, zinc, and manganese were constant in 2004. Interactions between site and harvest factors were usually absent except for calcium, boron, manganese, and copper in 2003 and iron in 2004. For those leaf traits that did differ between harvests, variation existed primarily between the early harvests (1 and 2) and later harvests (5 and 6) (Figure 1). Oftentimes, the initial leaf character value at harvest 1 was not significantly different from the final harvest value. For example, nitrogen in 2003 showed a high level at harvest 1, a significantly lower level at harvest 5, and a return to its high level at harvest 7. Similar dips in nutrient levels at mid-harvests were seen in phosphorus and potassium in 2004. Other nutrients that showed numerical but non-significant differences (p-value < 0.1) at mid-date harvests were nitrogen in 2004 and magnesium in both 2003 and 2004. In 2003, Brix %, osmolality, and SPAD chlorophyll content all showed a significant effect of harvest date; Brix % and osmolality exhibited increasing levels as harvest date progressed, while SPAD chlorophyll reached its highest level at harvest 3 and continued to decrease with subsequent harvests (data not shown).
Correlation and Regression of Leaf Characters With Maturity
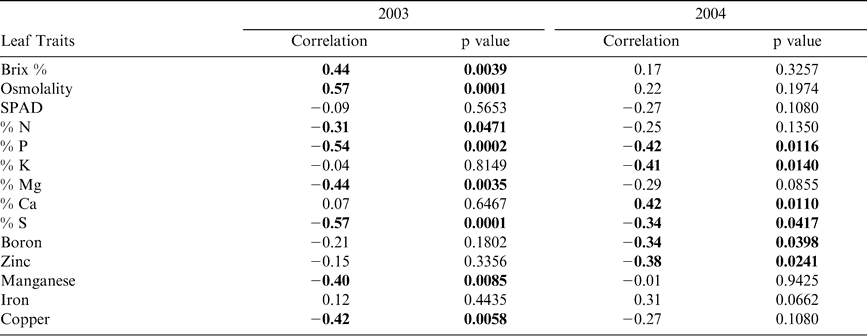
Pearson correlation analysis revealed several leaf characteristics were highly correlated with Maturity Index 1 in both 2003 and 2004 when tested across sites (Table 5). In 2003, Brix %, osmolality, and percent nitrogen, magnesium, manganese, and copper were correlated with crop maturity but were not correlated in 2004. Alternatively, percent potassium, calcium, and the concentration of boron and zinc were correlated with Maturity Index 1 in 2004 only. Percent phosphorus and sulfur were correlated with maturity in both years. All significant correlations between leaf nutrient contents and Maturity Index 1 were negative. Because Maturity Index 1 continued to increase with subsequent harvests (Figure 2), harvest number represented a level of crop maturity. The negative correlations of leaf nutrients with Maturity Index 1 indicate that the overall pattern in canopy nutrient content was a decline over time as the crop matured.
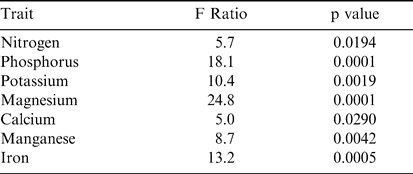
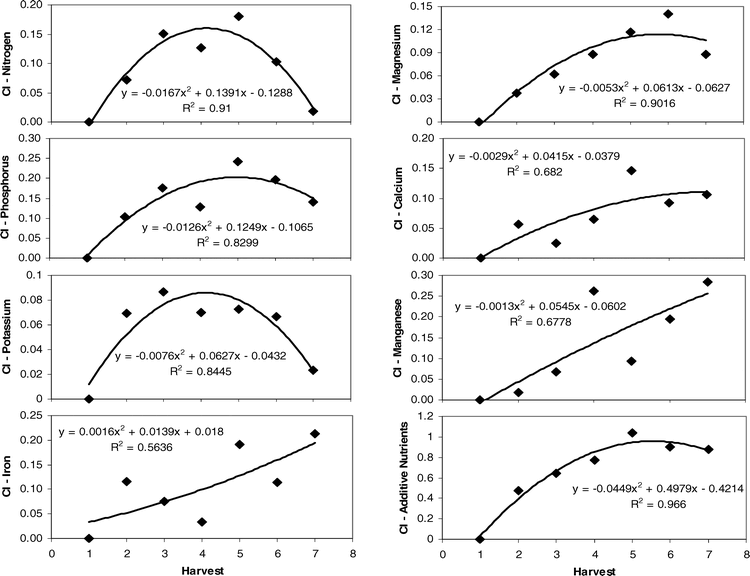
To determine those leaf traits that were the most important in influencing changes in Maturity Index 1 across both sites and years, stepwise regression was performed (Table 6). The results indicated that nitrogen, phosphorus, potassium, magnesium, calcium, manganese, and iron were significant in the regression model. When calculating the relative changes in the value of these key nutrients across harvests, the regression of the Change Index (CI) with harvest indicated most had parabolic patterns (Figure 3). R-squared values ranged from 0.56 for iron to 0.91 for nitrogen levels in leaf tissue when plotted against harvest number. When all CI values for these seven nutrients were summed at given harvest dates, the relationship with harvest was strongly parabolic with an improved R2 value of 0.97 (Figure 3). The pattern indicated that this additive nutrient value increased to harvest 5, leveled off by harvest 6, and began to drop after that point.
Stepwise regression results for leaf characteristics predicting Maturity Index 1. All measured traits were tested in the model and the resulting traits were found to have a significant effect on Maturity Index 1. Adjusted R2 of the model is 0.56. Model was performed across both sites (Sasser and Dawson) and both years (2003 and 2004).
Spectral Response Curves and Vegetation Indices
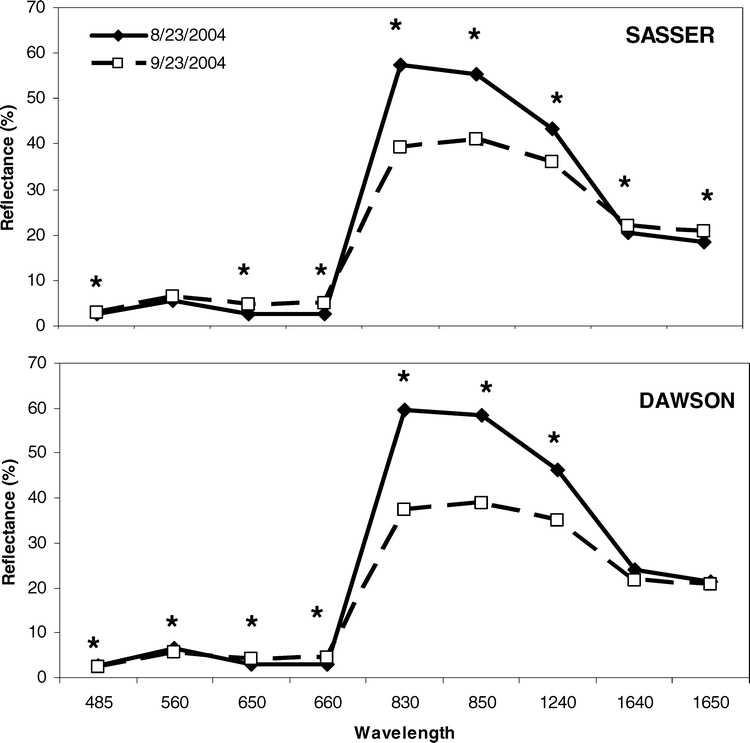
Spectral response curves (SRC) for each site and harvest in 2004 were constructed to evaluate the magnitude of change in reflectance patterns as the crop approached maturity. At each site, SRC collected coincident with the early harvest date (23 August 2004) exhibited a typical spectral response pattern (Figure 4). Specifically, spectra peaked in at 560 nm (green) and 830 nm (NIR), with the largest peak in the NIR. Beyond 830 nm, SRC gradually declined out to 1650 nm. Between 23 August and 23 September 2004, significant differences in spectral response patterns were observed at each site; at Dawson, differences in spectral response were observed throughout the visible and NIR spectrum. Changes in spectral response were most notable in the 830 and 850 nm bands, where spectral response decreased as much as 19 to 22 % between the August and September sampling times. At the Sasser site, significant differences in spectral response over time were also observed throughout the VIS and NIR (Figure 4). However, no significant differences between August and September were observed at 560 nm (green) at this study site. In the NIR, however, spectral response patterns resembled those observed at the Dawson site, decreasing from 14–18 % between the two dates.
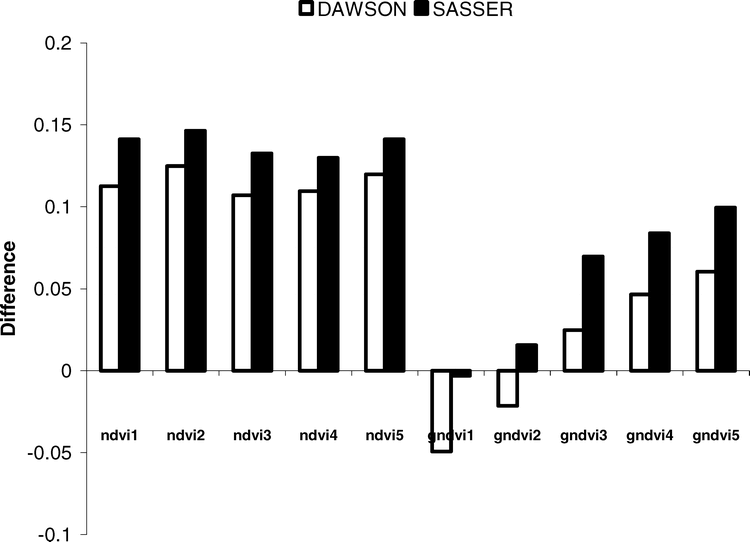
Ten different vegetation indices were evaluated as a means to infer peanut maturity. Five indices were calculated as a normalized ratio of NIR and red spectra (NDVI) and five indices were calculated as a normalized ratio of NIR and green spectra (GNDVI) (Table 3). At both study sites, significant differences in vegetation indices were observed over time. Perhaps most notably, differences in index values were greatest using a combination of NIR and red spectra. At the Dawson site, the NDVI values decreased over time having an overall change in response from 0.11 to 0.12 units, compared to only 0.05 to 0.06 units using the GNDVI's (Figure 5). A similar trend was observed at the Sasser study site, with the NDVI decreasing by as much as 0.13 to 0.15 units. However, at the Sasser site, not all GNDVI indices were significant. Only GNDVI indices utilizing NIR spectra between 830–1240 nm showed significant changes over time (0.07 to 0.10 units). This may be related to the lack of change in green spectra and relatively smaller changes in the 1640 and 1650 nm bandwidths (Figure 5). It appears that the NDVI utilizing a narrow band centered at 1640 (± 8 nm, NDVI2) or a wider band centered at 830 nm (± 70 nm, NDVI5) is most sensitive to changes in peanut maturity. Utilizing either NDVI2 or NDVI5 resulted in the greatest change in crop response over time at both study locations.
Discussion
The maturity process in peanut involves complex changes in assimilate transport and partitioning resulting in stabilization of protein, increases in oil, and decreases in carbohydrate content (Hung, 1994). The prediction of maturity in peanut is no more economically important than in other crops, but it presents more of a challenge to growers because the peanut is shielded in the ground away from view. The maturity profile board developed by Williams and Drexler (1981) has provided the industry with a useful and reliable method to determine peanut maturity; however the development of more objective methods that do not rely on sequential, destructive harvesting of pods may streamline the process. It seems reasonable that leaf biochemistry would reflect the peanut maturation process because leaves provide the developing pod with much of its carbohydrate and nutrient needs. This seems especially true in peanut whereby its relegation to the subsoil arena precludes a pod from photosynthesizing or providing any of its own assimilate requirement.
Rowland et al. (2006) identified a degree day method, based on the method Mills (1964) presented, which accurately predicted peanut maturity. However, if this method could be strengthened by combining it with an in-field assessment of crop maturity, a very robust, non-destructive prediction of digging date could be provided to growers. Many of the canopy traits measured in the current study appear to be promising candidates for predicting plant maturity because they exhibit changing levels during the late season corresponding with the latter stages of pod maturation. These included measures of solute content (Brix and osmolality), chlorophyll, and macro- and micro-nutrients. However, the consistency in the response of these characteristics across years is really paramount to their utility in being able to predict peanut maturity in a production setting. Even more important, the strength of their correlation with Maturity Index 1, representing the maturity level of the crop, was critical. The traits that held up to these standards represented a smaller group, namely the nitrogen, phosphorus, potassium, magnesium, calcium, manganese, and iron content of the leaves. The general pattern in these seven nutrients was a decrease in levels as harvest time progressed; this was especially true up to harvest 3 or 4. A decrease in leaf nutrient content during the latter part of the growing season is a common finding in many plant species including strawberry (Fragaria × ananassa), potato (Solanum tuberosum L.), olive (Olea europaea L.), rice (Oryza sativa L.), pecan (Carya illinoensis Wangenh. K. Koch), bromeliad (Werauhia sanguinolenta Linden ex Cogn. Marchal), and cotton (Bronson et al., 2001; Daugaard, 2001; Perica, 2001; Alva et al., 2002; Crozier et al., 2004; Fageria, 2004; Kim and Wetzstein, 2005; Zotz and Richter, 2006). These decreases can be due to either reproductive demands that cannot be matched by the plant, thereby leading to the mobilization of nutrients out of leaves, and/or the onset of leaf senescence (Perica, 2001; Alva et al., 2002; Kim and Wetzstein, 2005; Zotz and Richter, 2006). Alternatively, in the current study, leaf calcium showed increasing levels across harvests; a common pattern in strawberry and bromeliad leaves as well (Daugaard, 2001; Zotz and Richter, 2006). It was somewhat surprising that Brix level was not consistent across years in predicting maturity because pods should serve as a strong carbohydrate sink. Brix was found to actually increase with harvest date in 2003 (data not shown), indicating that carbohydrates were not limiting in this crop. This is a common pattern in some crops where nutrients instead of carbon are limiting during reproductive development (Zotz and Richter, 2006).
However, beyond harvest 3 or 4, the current study showed a recovery of many nutrients to their harvest 1 levels, resulting in a quadratic relationship in nutrient content from first to last harvest (Figure 1). This pattern has also been seen in other plants including rice, pecan, and cotton (Bronson et al., 2001; Fageria, 2004; Kim and Wetzstein, 2005). In the case of peanut, the increase in leaf nutrient levels at the latest harvest dates may reflect the degradation of the peg, and thus the pipeline of water and nutrients from the plant into the maturing pod. It has been shown that at 110 days after planting and beyond, nearly 95% of the moisture in the maturing pod is received from the surrounding soil and not from the plant itself via the peg (unpublished data). If water flow through the peg is cut off, nutrient transport is likely to be severely truncated at this time as well. This would allow the nutrient levels in the canopy to recover to previous levels, due to the continued assimilation and uptake of the plant with no subsequent export to the maturing crop.
It appears that the prediction of maturity can capitalize on this quadratic pattern in nutrient content across harvests. The calculation of the differences between harvest 1 levels and subsequent nutrient levels via the Change Index and the summation of these incremental changes in all of the predictive nutrients across sites and years showed a very strong relationship with harvest and thus Maturity Index 1. Overall, the additive Change Index increased over time with an eventual peak at the fifth or sixth harvest, followed by a slight dip in levels with subsequent harvests (Figure 3). What is even more promising is the fact that this additive Change Index may accurately predict the optimum economic return to the grower. While the maturity of the crop continued to increase across harvests in each year (Figure 2), yield and net value reached a peak after accumulation of approximately 2400 degree days and tapered off at the last harvests with additional degree day units (Rowland et al., 2006). This may be because yield may reach some maximum at a given degree day accumulation but then be counteracted by loss of extra-mature pods over time as maturity increases. Therefore, the calculation of the Change Index may accurately reflect the optimum balance between maturity and economic return.
Aside from nutrient status of the peanut canopy, spectral response of the canopy in 2004 was a very promising technique for predicting crop maturity. This was to be expected because spectral indices are often reflective of the nutrient status of many crops including corn, cotton, and wheat (Triticum aestivum L.) (Leamer et al., 1980; Wood et al., 1992; Blackmer et al., 1994; Osborne et al., 1994; Filella et al., 1995; Bausch and Duke, 1996; Barnes et al., 2000; Daughtry et al., 2000; Li et al., 2001; Osborne et al., 2002; Strachan et al., 2002; Bronson et al., 2003; Sullivan et al., 2004). In the present study, spectra peaked at 560 nm (green) and 830 nm (NIR), with the largest peak in the NIR. Spectral response in these regions is primarily associated with chlorophyll content in the green region, while NIR reflectance is more highly correlated with intercellular structure and water content (Hatfield, 1990; Hatfield and Pinter, 1993). Beyond 830 nm, spectral response curves gradually declined out to 1650 nm. In another study, Sullivan and Holbrook (2007) demonstrated a similar spectral response pattern over a peanut canopy prior to inducing drought. However, no significant differences were observed at 560 nm (green) at Sasser. This is a key difference between sites and reflects the stable chlorophyll concentrations as the crop reaches maturity. Thus, green spectra may not be particularly well-suited for determining peanut maturity.
Vegetation indices consisting of a normalized ratio of either red and NIR (NDVI) or green and NIR (GNDVI) spectra were used as a discrete measure of changes in the peanut canopy as the crop matured. While the use of vegetative indices is not new, only one study has demonstrated the relationship between peanut yield and the NDVI or GNDVI (Sullivan and Holbrook, 2007); and only one has examined the relationship between these indices for estimating peanut crop maturity in the U.S., but found no conclusive pattern (Sanders et al., 2002). In the current study, compared to the GNDVI, the NDVI proved most sensitive to changes in canopy reflectance as the peanut crop approached maturity. Specifically, an NDVI utilizing either a narrow band centered on 1640 nm or a wide band centered on 830 nm were best, decreasing as the crop approached maturity.
Although these results certainly show promise in the southeastern U.S., caution should be used in applying these methods to other peanut producing regions with varying climates until further testing is conducted. Also, in areas of west Texas and the Virginia-Carolina U.S. region, the selection of optimum harvest times may be less important because of shorter growing seasons and the often limited opportunity to delay harvest regardless of the maturity of the crop.
In conclusion, it appears that the combination of degree day calculations and nutrient monitoring during the last 30 days before the maximum reported season length for a given peanut cultivar provides an accurate, non-destructive method for predicting harvest date in the southeastern U.S. Rowland et al. (2006) showed the predictive capability of the Mills (1964) degree day method adjusted for cumulative water received (irrigation or precipitation) over the growing season was close to 92%. This degree day calculation can alert the grower when it is nearly time to dig and assist in scheduling the order of harvest among multiple fields. For more precise harvest predictions, canopy nutrient analysis and the calculation of the Change Index show predictive success not only with the maturity of the crop but with the economic return to the grower. Therefore, the following recommendation could be provided to southeastern growers interested in using a non-destructive method for predicting harvest dates: 1) calculate the Mills (1964) degree day method modified by cumulative water received by the crop and attempt to accumulate 2400 DD before harvest; and 2) at 100 days after planting, analyze 15–20 second nodal leaves for nutrients and repeat 30, 34, 38, and 42 days after the initial testing. Once a peak and subsequent drop in additive CI has occurred and 2400 DD have been accumulated, harvest would be recommended. To strengthen and make harvest predictions logistically easier, further development of spectral indices as predictors is needed. These indices appear to be very promising and may become more feasible to growers as remote sensing technologies become increasingly available.
Acknowledgements
We thank Larry Powell, Kathy Gray, and Manuel Hall whose technical expertise made the project possible. We credit L. Powell with the consideration and development of degree day model tests.
Literature Cited
Alva A. K. , Hodges T. , Boydston R. A. , and Collins H. P. 2002 Dry matter and nitrogen accumulations and partitioning in two potato cultivars. J. of Plant Nutrition 25 : 621 – 1630 .
Andrade F. H. , Otegui M. E. , and Vega C. 2000 Intercepted radiation at flowering and kernel number in maize. Agron. J 92 : 92 – 97 .
Barnes E. M. , Clarke T. R. , Richards S. E. , Colaizzi P. D. , Haberland H. , Kostrzewski M. , Waller P. , Choi C. , Riley E. , Thompson T. , Lascano R. J. , Li H. , and Moran M. S. 2000 Coincident detection of crop water stress, nitrogen status and canopy density using ground-based multispectral data. Proceedings of the 5th International Conference on Precision Agriculture, 16–19 July, Bloomington, Minnesota (American Society of Agronomy, Madison, Wisconsin), unpaginated CD-Rom
Bausch W. C. and Duke H. R. 1996 Remote sensing of plant nitrogen status in corn. Amer. Soc. of Agric. Engineers 39 : 1869 – 1875 .
Blackmer T. M. , Schepers J. S. , and Varvel G. E. 1994 Light reflectance compared with other nitrogen stress measurements in corn leaves. Agron. J 86 : 934 – 938 .
Bronson K. F. , Chua T. T. , Booker J. D. , Keeling J. W. , and Lascano R. J. 2003 In-season nitrogen status sensing in irrigated cotton: II. Leaf nitrogen and biomass. Soil Sci. Soc. Amer. J 67 : 1439 – 1448 .
Bronson K. F. , Onken A. B. , Keeling J. W. , Booker J. D. , and Torbert H. A. 2001 Nitrogen response in cotton as affected by tillage system and irrigation level. Soil Sci. Soc. Amer. J 65 : 1153 – 1163 .
Cober E. R. , Stewart D. W. , and Voldeng H. D. 2001 Photoperiod and temperature responses in early-maturing, near–isogenic soybean lines. Crop Sci 41 : 721 – 727 .
Crozier C. R. , Walls B. , Hardy D. H. , and Barnes J. S. 2004 Response of cotton to P and K soil fertility gradients in North Carolina. J. Cotton Sci 8 : 130 – 141 .
Daugaard H. 2001 Nutritional status of strawberry cultivars in organic production. J. Plant Nutrition 24 : 1337 – 1346 .
Daughtry C. S. T. , Walthall C. L. , Kim M. S. , Brown de Colstoun E. , and McMurtrey J. E. 2000 Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sensing of Environ 74 : 22 – 239 .
Dufault R. J. 1997 Determining heat unit requirements for broccoli harvest in coastal South Carolina. J. Amer. Soc. Hort. Sci 122 : 169 – 174 .
Fageria N. K. 2004 Dry matter yield and nutrient uptake by lowland rice at different growth stages. J. Plant Nutrition 27 : 947 – 958 .
Filella I. , Serrano L. , Serra J. , and Penuelas J. 1995 Evaluating wheat nitrogen status with canopy reflectance indices and discriminant analysis. Crop Sci 35 : 1400 – 1405 .
Fincher P. G. , Young C. T. , Wynne J. C. , and Perry A. 1980 Adaptability of the arginine maturity index method to Virginia type peanuts in North Carolina. Peanut Sci 7 : 83 – 87 .
Gitelson A. A. , Kaufman Y. J. , and Merzlyak M. N. 1996 Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sensing of Environ 58 : 289 – 298 .
Harrison K. and Tyson A. 1993 Irrigation scheduling methods. Bulletin no. B-974 Ga. Agric. Exp. Stn., Athens, GA.
Hatfield J. L. 1990 Remote detection of crop stress: application to plant pathology. Phytopathol 80 : 37 – 39 .
Hatfield J. L. and Pinter P. J. 1993 Remote sensing for crop protection. Crop Protect 12 : 403 – 412 .
Hung Y. C. 1994 Effect of harvest date on the chemical composition of peanuts. Trans. of the ASAE 37 : 1287 – 1291 .
Jensen M. E. and Haise H. R. 1963 Estimating evapotranspiration from solar radiation. J. Irrig. Drainage Div. ASCE 89 : 15 – 41 .
Kim T. and Wetzstein H. Y. 2005 Seasonal fluctuations in nutrients and carbohydrates in pecan leaves and stems. J. Hort. Sci. Biotech 80 : 681 – 688 .
Lamb M. C. , Masters M. H. , Rowland D. , Sorensen R. B. , Zhu H. , Blankenship P. D. , and Butts C. L. 2004 Impact of sprinkler irrigation amount and rotation on peanut yield. Peanut Sci 31 : 108 – 113 .
Leamer R. W. , Noriega J. R. , and Gerbermann A. H. 1980 Reflectance of wheat cultivars as related to physiological growth stages. Agronomy J 72 : 1029 – 1032 .
Li H. , Lascano R. J. , Barnes E. M. , Booker J. , Wilson L. T. , Bronson K. F. , and Segarra E. 2001 Multispectral reflectance of cotton related to plant growth, soil water and texture, and site elevation. Agronomy J 93 : 1327 – 1337 .
Mills W. T. 1964 Heat unit system for predicting optimum peanut-harvesting time. Trans. of the ASAE 7 . 307-309, 312
Monje O. A. and Bugbee B. 1992 Inherent limitations of nondestructive chlorophyll meters: a comparison of two types of meters. Hortsci 27 : 69 – 71 .
NeSmith D. S. and Hoogenboom G. 1994 Variation in the onset of flowering of summer squash as a function of days and heat units. J. Amer. Soc. Hort. Sci 119 : 249 – 252 .
Osborne S. L. , Schepers J. S. , Francis D. D. , and Schlemmer M. R. 2002 Use of spectral radiance to estimate in-season biomass and grain yield in nitrogen - and water-stressed corn. Crop Sci 42 : 165 – 171 .
Osborne S. L. , Schepers J. S. , Francis D. D. , and Schlemmer M. R. 1994 Detection of phosphorus and nitrogen deficiencies in corn using spectral radiance measurements. Agronomy J 94 : 1215 – 1221 .
Perica S. 2001 Seasonal fluctuation and intracanopy variation in leaf nitrogen level in olive. J. of Plant Nutrition 24 : 779 – 787 .
Perry K. B. , Wu Y. , Sanders D. C. , Garrett J. T. , Decoteau D. R. , Nagata R. T. , Dufault R. J. , Batal K. D. , Granberry D. M. , and Mclaurin W. J. 1997 Heat units to predict tomato harvest in the southeast USA. Agric. and Forest Meteorology 84 : 249 – 254 .
Rouse J. W. , Haas J. R. H. , Schell J. A. , and Deering D. W. 1974 In Proc. ERTS-1 Symp., Greenbelt, MD. 10–15 Dec. 1974. NASA, Washington, D.C. 1974. Monitoring vegetation systems in the Great Plains with ERTS. 309 – 317 .
Rowland D. L. , Sorensen R. B. , Butts C. L. , and Faircloth W. H. 2006 Determination of maturity and degree day indices and their success in predicting peanut maturity. Peanut Sci 33 : 125 – 136 .
Sanders J. C. , Reynolds D. B. , Wilson D. G. , and Bruce L. M. 2002 Utilizing remote sensing to determine crop maturity. Proc. South. Weed Sci. Soc 55 : 147 .
Sanders T. H. , Lansden J. A. , Greene R. L. , Drexler J. S. , and Williams E. J. 1982a Oil characteristics of peanut fruit separated by a nondestructive maturity classification method. Peanut Sci 9 : 20 – 23 .
Sanders T. H. , Schubert A. M. , and Pattee H. E. 1982b Maturity methodology and postharvest physiology. 624 – 654 In Pattee H. E. and Young C. T. Peanut Science and Technology Yoakum, TX, USA Amer. Peanut Res. Educ. Society., Inc .
SAS 1997 Cary, NC, USA, SAS Institute Inc.
Spano D. , Cesaraccio C. , Duce P. , and Zinder R. L. 1999 Phenological stages of natural species and their use as climate indicators. Int. J. Biometeorol 42 : 124 – 133 .
Strachan I. B. , Pattey E. , and Boisvert J. B. 2002 Impact of nitrogen and environmental conditions on corn as detected by hyperspectral reflectance. Remote Sensing of Environ 80 : 213 – 244 .
Sullivan D. G. and Holbrook C. C. 2007 Using ground-based reflectance measurements as selection criterion for drought and aflatoxin resistant peanut genotypes. Crop Sci 47 : 423 – 432 .
Sullivan D. G. , Shaw J. N. , Mask P. , Rickman D. , Luvall J. , and Wersinger J. M. 2004 Evaluating corn (Zea mays L.) N variability via remote sensed data. Communications in Soil Science And Plant Analysis 35 : 2465 – 2483 .
USDA 2006 Peanut grading and testing. Online: http//:www.ars.usda.gov/main/doc.htmdocid3566. Washington, D.C.
Viator R. P. , Nuti R. C. , Edmisten K. L. , and Wells R. 2005 Predicting cotton boll maturation period using degree days and other climatic factors. Agronomy J 97 : 494 – 499 .
Williams E. J. and Drexler J. S. 1981 A non-destructive method for determining peanut pod maturity. Peanut Sci 8 : 134 – 141 .
Wood C. W. , Reeves D. W. , Duffield R. R. , and Edmisten K. L. 1992 Field chlorophyll measurements for evaluation of corn nitrogen status. J. Plant Nutrition 15 : 487 – 500 .
Young J. H. , Person N. K. , Donald J. O. , and Mayfield W. D. 1982 Harvesting, curing and energy utilization. 458 – 485 In Pattee H. E. and Young C. T. Peanut Science and Technology Yoakum, TX, USA Amer. Peanut Res. Educ. Society., Inc .
Zotz G. and Richter A. 2006 Changes in carbohydrate and nutrient contents throughout a reproductive cycle indicate that phosphorus is a limiting nutrient in the epiphytic bromeliad, Werauhia sanguinolenta. Annals of Botany 97 : 745 – 754 .
Notes
- USDA-ARS, National Peanut Research Laboratory, P.O. Box 509, 1011 Forrester Dr. SE, Dawson, GA 39842 [^]
- USDA-ARS, Southeast Watershed Research Laboratory, 2316 Rainwater Rd., Coastal Plain Experiment Station, Tifton, GA 31794 [^] * Corresponding author (email: diane.rowland@ars.usda.gov)
Disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Author Affiliations